Résumés
Abstract
Tetranychus urticae Koch (two-spotted spider mite) is an agricultural pest with a host range of over 1100 species of plants. Tetranychus urticae has rapidly developed resistance to a variety of synthetic chemical pesticides due to its high fecundity and short generation time. Plant essential oils have been recognized as a novel natural source of pest control that have a reduced impact to the environment and human health compared to synthetic pesticide application, and which may provide a viable alternative for managing T. urticae. The present study assessed the potential of a plant-derived product (product 102) as an acaricide, through topical and residual bioassays on a variety of plant species including common bean plant (Phaseolus vulgaris L.), lettuce (Lactuca sativa L.), tomato (Solanum lycopersicum L.), kale (Brassica oleracea L.), cucumber (Cucumis sativus L.), hops (Humulus lupulus L.) and hemp (Cannabis sativa L.). The results of our study indicate that C. sativa is not a suitable plant to host T. urticae. Product 102 was determined to be effective at preventing the growth of two known fungal species of economic concern (Cladosporium herbarum Persoon and Botrytis cinerea Persoon). By conducting acute contact toxicity tests, we also determined that product 102 is significantly less toxic to Bombus impatiens Cresson compared to the commonly used synthetic insecticide imidacloprid.
Keywords:
- bumblebees,
- essential oils,
- fungicide,
- topical toxicity,
- two-spotted spider mite
Résumé
Tetranychus urticae Koch (tétranyque à deux points) est un ravageur agricole ayant plus de 1100 espèces de plantes hôtes. Tetranychus urticae a rapidement développé une résistance à une variété de pesticides chimiques synthétiques en raison de sa fécondité élevée et de son temps de génération court. Les huiles essentielles de plantes ont été reconnues comme une nouvelle source naturelle de lutte contre les ravageurs ayant un impact moindre sur l’environnement et la santé humaine par rapport à l’application de pesticides synthétiques, et qui peuvent constituer une alternative viable pour lutter contre T. urticae. La présente étude a évalué le potentiel d’un produit dérivé d’une plante (produit 102) en tant qu’acaricide, par le biais d’essais biologiques topiques et résiduels sur une variété d’espèces végétales, y compris le haricot commun (Phaseolus vulgaris L.), la laitue (Lactuca sativa L.), la tomate (Solanum lycopersicum L.), le chou (Brassica oleracea L.), le concombre (Cucumis sativus L.), le houblon (Humulus lupulus L.) et le chanvre (Cannabis sativa L.). Les résultats de notre étude indiquent que C. sativa n’est pas une plante appropriée pour T. urticae. Le produit 102 s’est avéré efficace pour empêcher la croissance de deux espèces fongiques connues et préoccupantes sur le plan économique (Cladosporium herbarum Persoon et Botrytis cinerea Persoon). En effectuant des tests de toxicité aiguë par contact, nous avons également déterminé que le produit 102 est significativement moins toxique pour Bombus impatiens Cresson que l’insecticide synthétique couramment utilisé, l’imidaclopride.
Mots-clés :
- bourdons,
- huiles essentielles,
- fongicide,
- toxicité topique,
- tétranyque à deux points
Corps de l’article
INTRODUCTION
Natural based products are an excellent alternative to synthetic pesticides (A.K. Tripathi et al. 2009). Plant essential oils have been recognized as a viable natural source of pesticide that reduce the impact to the environment and human health compared to synthetic products (A.K. Tripathi et al. 2009). Unlike many synthetic pesticides, essential oils are relatively nontoxic to mammals, and non-persistent in the environment. Given the potential essential oils have in controlling pest species and being eco-friendly alternatives to synthetic pesticides, they have been increasingly used in commercial pesticides (Bakkali et al. 2008; Fierascu et al. 2020; Isman 2000, 2006; Isman and Machial 2006; Raveau et al. 2020; Regnault-Roger et al. 2012).
Tetranychus urticae Koch (Trombidiformes: Tetranychidae) (two-spotted spider mite) is an agricultural pest that has developed resistance to many pesticides and has a host range of over 1100 species of plants (Xu et al. 2018). Mites can rapidly evolve resistance to many synthetic pest control products due to their high fecundity and short generation time (Grbic et al. 2007). Plant-derived products are a viable alternative to synthetic pesticides and should be considered for the mana-gement of T. urticae.
Botrytis cinerea Persoon is a ubiquitous fungus responsible for grey mould on many economically important crops including vegetables (i.e., tomato, cucumber, and lettuce), ornamentals (i.e., roses and gerbera), bulbs (i.e., onions) and fruits (i.e., grapevine, strawberry, and kiwifruit) (Leroux 2007). For the past century, control of B. cinerea has relied heavily on synthetic fungicides. However, this is not regarded as a sustainable solution, as B. cinerea has shown rapid development of resistance to a variety of synthetic products (Elmer and Reglinski 2006). P. Tripathi et al. (2008) tested 26 essential oils against B. cinera, and among them Chenopodium ambrosioides, Eucalyptus citriodora, Eupatorium cannabinum, Lawsonia inermis, Ocimum canum, O. gratissimum, O. sanctum, Prunus persica, Zingiber cassumunar and Z. officinale exhibited the highest level of fungitoxic activity with 100% growth inhibition under laboratory conditions. Additionally, the study found that the antifungal potency of these oils was greater than that of some synthetic fungicides showing promising application of essential oils as fungicides.
Cladosporium herbarum Persoon is one of the most common environmental fungi to be isolated globally. The species occurs on herbaceous and woody plants and has frequently been isolated from air (Samson et al. 2000). Cladosporium herbarum is commonly associated with seeds of crops, including the common bean (Phaseolus vulgaris L.) (Dhingra et al. 2002). Cladosporium herbarum is not a pathogenic fungus, but it is of concern for human health being a known cause of asthma (Dhingra et al. 2002). Treatment of the seeds with fungicides can help eliminate pathogens and protect seedlings against various fungal diseases (Carvalho et al. 2011). Given that synthetic fungicides have a negative impact on the environment and human health, there is a need to develop more plant-derived fungicides (e.g., fungus killing) and fungistatic (e.g., fungus preventing) products (A.K. Tripathi et al. 2009).
In addition to testing the effectiveness of plant-derived products on pathogenic fungal species, it is important to examine the secondary effects on beneficial insects such as Bombus impatiens Cresson (Hymenoptera: Apidae) (bumblebees). Pollinators have been a key component of agriculture for centuries, with approximately 35% of human crops depending directly on pollinators (Codling et al. 2016; Klein et al. 2006). In recent years there have been increasing reports of overwintering losses of bee colonies and challenges in maintaining healthy colonies globally. This impact to bee population can be attributed to several factors, such as changes in climate, genetics, changes in available nutritional sources, parasites, and viruses. Recent studies suggest that one other factor may be the use of pesticides (Codling et al. 2016; Fairbrother et al. 2014).
Neonicotinoids are one of the most widely used classes of pesticides. The extensive use of neonicotinoids is mainly because they exhibit greater toxicity to invertebrates compared with vertebrates. Neonicotinoids are water soluble but show low toxicity toward fish. This class of pesticide is also seen as convenient because it is persistent in the environment, requiring less additional spraying, and it is versatile in the mode of application (Bonmatin et al. 2015; Codling et al. 2016). However, neonicotinoids accumulate in plant tissues, including pollen. Yamada et al. (2012) examined toxicity effects of common neonicotinoids and determined that concentrations as little as 1000 ng mL-1 of clothianidin and 400 ng mL-1 of dinitrofuran administered to multiple hives caused colony collapse disorder (CCD) or pre-CCD behaviour. Given the harmful impact neonicotinoids have on pollinators, there is a growing interest and urgency in adopting natural-based pesticides.
In this work, we have tested the properties of two novel essential oil-based products develop by a Canadian company (called ‘product 101’ and ‘product 102’) on different model pest species, looking at their potential as pesticides, fungicides, and the potential negative effects on non-target insect pollinators. Our study conducted a variety of acaricide experiments to test products 101 and 102 against T. urticae. Additionally, we performed fungistatic and fungicide experiments against C. herbarum and B. cinerea. Finally, we performed acute contact toxicity tests involving the essential oil-based product 102 on B. impatiens.
MATERIALS AND METHODS
Animals and plant care
Spider mites were provided by Vineland Research and Innovation Centre (Vineland Station, ON, Canada) to establish a colony at Acadia University, Wolfville (NS, Canada). The mite colony was maintained on common bean plants (Phaseolus vulgaris L.) in wire mesh cages (100 x 100 x 100 cm) located in a growth chamber (25 ± 2 °C, 16:8 L:D, 70 ± 5% RH). Bean plants were changed every 2 days, such that several of the oldest plants were replaced with 3-week-old plants. Repla-cement of bean plants provided a continuous food supply for the mite colony.
All plants involved in this study, including common bean plant (Phaseolus vulgaris L. var. ‘Red Kidney’), lettuce (Lactuca sativa L. var. ‘Little Gem Pearl’), tomato (Solanum lycopersicum L. var. ‘Scotia’), kale (Brassica oleracea L. var. ‘Darkibor’), cucumber (Cucumis sativus L. var ‘Summer dance’), hops (Humulus lupulus L. var. ‘Fuggle’) and hemp (Cannabis sativa L. var. ‘CFX-2’), were grown in phytotrons in the Harriet Irving Botanical Gardens (K.C. Irving Environmental Science Centre, Acadia University, NS, Canada), kept between 18 and 25 °C on a 12:12 L:D photoperiod. Plants were used in experiments when between 3 and 5-weeks old.
All Bombus impatiens Cresson (Hymenoptera: Apidae) obtained for this study were purchased from Koppert Biological Systems (Koppert Canada Limited, ON, Canada). All bees were selected from a single colony to provide similar origin and health. Bees provided from Koppert Biological Systems contained a queen, workers, and brood. The largest bees were selected for the study, and the bees were used within 6-weeks of arriving at Acadia University. The colony was maintained in a growth chamber (25 ± 2 °C, 16:8 L:D, 70 ± 5% RH). For nutrients, the colony was given 1 tsp of pollen balls daily (Hawkins Honey, ON, Canada), and cotton balls (Walmart Canada, Mississauga, ON, Canada) saturated with sugar solution (1:4 sugar water ratio) (Redpath Sugar Ltd, Toronto, ON, Canada) were replaced daily.
Chemicals
Products 101 and 102 were provided by Nutrilife Plant Products Limited (Abbotsford, BC, Canada). Products 101 and 102 were prepared at a 1:50 dilution according to the manufacturer’s specifications. Product 102 is an experimental formulation adapted from Nutrilife SM-90 multi-purpose wetting agent, in combination with a coriander essential oil active ingredient. Product 102 has sulphonated castor oil, while product 101 has sulphonated canola oil, and both products have coriander seed oil. The specific amount of each component of the product 101 and 102 formulations cannot be disclosed since the formulations are company proprietary information. The product is under registration and it will be disclosed at the right time under the company’s conditions. Vegol (Vegol® Crop Oil, Neudorff Commercial Canada, BC, Canada) was used as positive control in pesticide assays and was prepared at 1:50 concentration according to label recommendations. Green Earth Concentrate Lime Sulphur Insecticide-Fungicide Solution (Brantford, ON, Canada) was used as positive control in antifungal assays and was prepared at a 1:10 concentration based on label recommendations. Imidacloprid (positive control in pollinator experiments) was purchased from Sigma-Aldrich (Saint-Louis, MO, USA).
Acaricidal testing of products 101 and 102
Acaricidal testing of products 101 and 102 was conducted using both topical and residual toxicity tests, using a newly developed protocol. In both topical and residual testing, four different products were used: 1) control/water, 2) Vegol (1:50 dilution), 3) product 101 (1:50 dilution), 4) product 102 (1:50 dilution). Topical toxicity was tested on leaves from lettuce, tomato, kale, cucumber, hops and hemp plants. Residual toxicity was tested on leaves from hops, hemp, and bean plants.
For topical experiments, the mites were sprayed directly after being transferred onto the foliage, and mortality was assessed at 1, 2, 4, and 24 h time intervals. For residual experiments, foliage was sprayed prior to transferring the mites. Mites were transferred at 2, 24, 48, and 72 h post-treatment, and mortality was assessed at 24 h after transferring. A dissecting microscope was used (AmScope SM-1BSX-64S, Irvine, CA, USA) to assess for movement of mites when probed with a paintbrush to determine mortality. For the topical experiments, there were 5 mites per treatment type and the experiment was repeated 5 times (n = 25). For the residual experiments, there were 10 mites per treatment type and the experiment was repeated 5 times (n = 50). All mites involved in acaricidal studies were mature adults.
Preparing tomato juice agar plates
Agar plates were prepared (Gonzalez et al. 2020; He et al. 2011) within one week of conducting experiments. Agar solution consisted of one part tomato juice (Heinz, ON, Canada), four parts distilled water, and 2% (w/v) agar (Cape Crystal Brands, New Jersey, USA). Agar solution was brought to a boil on a hot plate and boiled for 5 min. The solution was cooled for one minute prior to pouring into plastic Petri dishes (100 mm x 15 mm) (FisherbrandTM, Fisher Scientific, ON, Canada). Approximately 20 mL were poured into each plate and left to cool for 30 min. Then the plates were sealed with Parafilm (FisherbrandTM, Fisher Scientific, ON, Canada) and stored in a fridge at 4 °C until the start of experiments.
Testing efficacy as a fungicide
To test the efficacy of product 102 as a fungicide, two different fungi were investigated: Botrytis cinerea Persoon and Cladosporium herbarum Persoon. For the fungicidal studies, we focused on product 102 as preliminary studies using 102 demonstrated it was a more effective acaricide. For both species of fungi, spores were plated and grown for 4 days in an incubator (25 ± 2 °C; 90% humidity) (Gonzalez et al. 2020; He et al. 2011). Cladosporium herbarum spores were obtained from purchased strawberries, and the collected spores were sequenced to confirm identification. Botrytis cinerea spores were provided by the Walker mycology lab at Acadia University (Wolfville, NS).
The fungal experiment testing efficacy of product 102 was conducted using two different approaches described by He et al. (2011) and Gonzalez et al. (2020): (1) inoculating the plates after treatment to test for the prevention of fungus growth; (2) inoculating the plates prior to treatment to test for the killing of fungus. To inoculate agar plates with fungus, a sterile cotton swab (Puritan Medical Products, Guilford, Maine, USA) was swiped for approximately 5 s on fungus culture and then T-streaked onto clean agar plates. Inoculation of plates was done in a fume hood, and we included a negative control group in our experimental design to help assess for any potential contamination.
For both experiments, there were 24 replicates per treatment group (i.e., product 102, reference fungicide group, and control groups). Each experiment included a positive and a negative control. The positive control plates were treated with distilled water and inoculated with fungus to assess normal fungus growth rate. The negative control plates were treated with distilled water, but not inoculated with fungus, to test for any potential contamination introduced during the experimental protocol. The treatment group of interest was product 102 (1:50 dilution), which was compared to a commercially sold reference fungicide (Green Earth Concentrate Lime Sulphur Insecticide-Fungicide Solution; Brantford, ON, Canada) (1:10 dilution).
When testing fungal growth inhibition, plates were treated with water, product 102, or the Green Earth product, and left to absorb the treatment for 1 h. The plates were treated in a fume hood, and we left the plates half covered with lids until inoculation. To treat plates, a spray bottle (Silgan plastics, CO, USA) contained each of the different treatments and a single spray application (approximately 1 mL) was given to each plate. We chose to treat the plates as described because we wanted to recreate normal application conditions. Then the plates were inoculated with fungus (except for negative control), sealed with Parafilm, and placed in the incubator (25 ± 2 °C; 90% humidity). Plates were checked daily for 7 days following inoculation. Approximate percent fungus cover was recorded daily for each plate. To measure percent cover, we took a photo of each plate, and then a 1 cm-grid was used to help visually assess percent fungus cover on a computer.
When testing fungicidal activity, all plates except the negative control group were inoculated with fungus and placed in incubator for 4 days to allow for fungal growth. After 4 days (all plates had at least 50% cover), plates were treated with product 102, Green Earth product, or water. Plates were then resealed in Parafilm and placed in incubator. Plates were checked daily for 7 days following treatment, and percent fungus cover was recorded.
Impact of product 102 on Bombus impatiens mortality
To determine the effects of product 102 on B. impatiens, contact toxicity tests were performed following a protocol adapted from Medrzycki et al. (2013). For each experiment replication, there were 5 bees for each treatment group, and a total of 5 replicates were run (n = 25). Product 102 was tested at 5 different concentrations (0.1:50, 0.5:50, 1:50, 5:50, 10:50). Imidacloprid was used as the positive control group at 2 ng μL-1. We applied 10 μL of the imidacloprid solution for a total amount of 20 ng, which is the determined topical LD50 for a bumblebee species (Bombus terrestris) (Marletto et al. 2003). Distilled water was used as negative control.
Bees were individually picked from the colony by using forceps and grabbing them by a back leg, and then placing a single bee in a 50 mL Falcon tube (FisherbrandTM, Fisher Scientific, ON, Canada). Prior to treatment, each bee was anaesthetized using carbon dioxide, where just enough carbon dioxide was blown into the Falcon tube to arrest the movement of the individual bee. The anaesthetized bee was immediately placed on a glass Petri dish (Corning Inc., Arizona, USA), and the Petri dish was placed in a polystyrene foam box containing crushed ice to minimize movement during treatment. Then 10 μL of the assigned treatment solution was applied to the thorax of the bee using a micropipette.
Treated bees were kept in well-ventilated wooden cages (20.5 x 15 x 13.5 cm) with a syringe of 5 mL of sugar water (1:1 sugar water ratio). All wooden cages were kept in a growth chamber (25 ± 2 °C, 16:8 L:D, 70 ± 5% RH). Bees with different treatments were kept in separate boxes and there was a total of 5 bees per box. Mortality was recorded at 4, 24 and 48 h after treatment.
Statistics
All statistical analyses were done using R version 4.0.3 (R Core Team 2020).
We ran a generalized linear model (glm) to assess if plant type, treatment, and/or time had a significant impact on mite mortality for both topical and residual toxicity tests (α = 0.05).
We performed a Shapiro-Wilk normality test on the fungus growth rate for each treatment group, and determined the data were not normally distributed. Therefore, to analyze whether fungus growth rate was significantly different among treatment groups, a Kruskal-Wallis rank sum test was performed for each of the different fungus experiments, followed by a Dunn multiple comparison with the Bonferroni method (α = 0.05). These statistical tests were chosen given that the data were not normally distributed.
To determine if the concentration of product 102 signi-ficantly impacts mortality of bees at 4 h, the data for bee mortality at 4 h was fitted to a binomial model with Probit link. Additionally, LD50 of product 102 at 4 h was calculated with Probit analysis. The same statistical analyses were performed for bee mortality at 24 h and 48 h.
To determine if time and concentration significantly impact bee mortality, the data were fitted to a linear mixed model by maximum likelihood.
Results
Topical toxicity
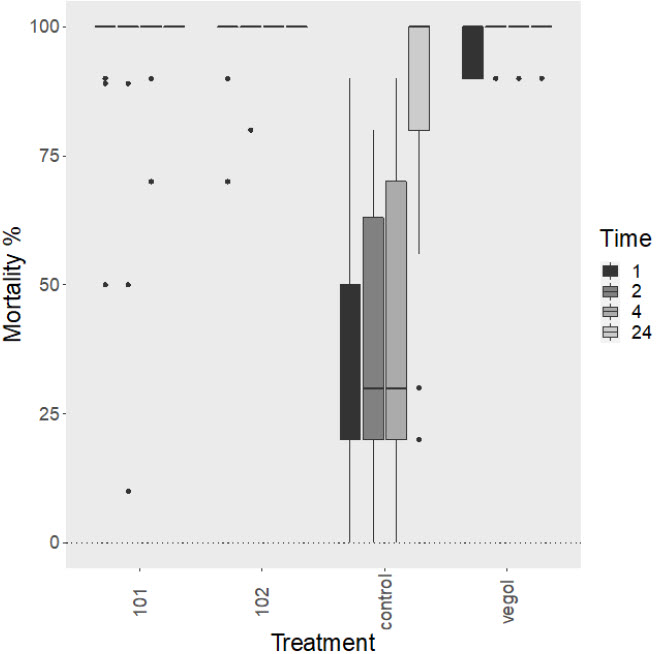
No significant difference in mite mortality for different treatment types was observed (Fig. 1). Interestingly, hemp (C. sativa) plants significantly impacted mite mortality as determined from the generalized linear model (t = 12.38; Pr(>|t|) < 0.001). Additionally, a significant difference in mite mortality for the control group on hemp compared to the control group on other plant species (t = -5.11; Pr(>|t|) < 0.001) was detected. The average mite mortality was higher in the control group on hemp plants compared to other plant species (Fig. 1). Time at 4 h post-treatment significantly impacted mite mortality (t = 3.04; Pr(>|t|) < 0.01) and at 24 h (t = 4.34; Pr(>|t|) < 0.001) (Fig. 2).
Figure 1
Mortality of Tetranychus urticae following topical treatment of Nutrilife product 101 and 102, control (water), and Vegol® Crop Oil on a variety of plant species (cucumber, hemp, hops, kale, lettuce, tomato), where mortality was recorded at 1hr, 2 h, 4 h, and 24 h post-treatment
For each plant species tested, n = 5, except for hemp (n = 25). The bar represents the median, the box represents the interquartile range, and the whiskers represent the maximum and minimum values that are not outliers.
Figure 2
Mortality of Tetranychus urticae following topical treatment of Nutrilife product 101 and 102, control (water), and Vegol® Crop Oil on hemp (Cannabis sativa), where mortality was recorded at 1 h, 2 h, 4 h, and 24 h post-treatment (n = 25)
The bar represents the median, the box represents the interquartile range, and the whiskers represent the maximum and minimum values that are not outliers.
Figure 3
Mortality of Tetranychus urticae following residual treatment of Nutrilife product 101 and 102, control (water), and Vegol® Crop Oil on a variety of plant species (bean, hemp, and hops), where mites were transferred at 2 h, 24 h, 48 h, and 72 h post-treatment
Mortality was recorded 24 h after transferring mites. For each plant species tested, n = 50. The bar represents the median, the box represents the interquartile range, and the whiskers represent the maximum and minimum values that are not outliers.
Table 1
Dunn multiple comparison with the Bonferroni method for growth rate of Cladosporium herbarum and Botrytis cinerea for each treatment group as recorded for 7 days following a pre-inoculation treatment, where prevention of fungal growth was determined (n = 24)
Residual toxicity
Overall, we detected no significant difference in mite mortality for treatment types or time (Fig. 3). Vegol applied on hops plants significantly impacted mite mortality (t = 2.07; Pr(>|t|) < 0.05). Mite mortality for Vegol was higher on average for residual tests performed on hops (Fig. 3).
Efficiency of product 102 as a fungicide
There was a significant difference in growth rate between treatment groups for C. herbarum growth inhibition (Kruskal-Wallis rank sum test; X2 = 440.84; DF = 3; P < 0.0001). The growth rate of the positive control group was significantly higher than the growth rate of the negative control group, Product 102, and Green Earth (Table 1; Fig. 4A).
Figure 4
Growth rate of Cladosporium herbarum and Botrytis cinerea for each treatment group as recorded for 7 days following a pre- or post-inoculation treatment, where prevention of fungus growth or killing of fungus was determined (n = 24)
(A): The prevention of Cladosporium herbarum growth. (B): The killing of Cladosporium herbarum. (C): The prevention of Botrytis cinerea growth. (D): The killing of Botrytis cinerea. The bar represents the median, the box represents the interquartile range, and the whiskers represent the maximum and minimum values that are not outliers.
Table 2
Dunn multiple comparison with the Bonferroni method for growth rate of Cladosporium herbarum and Botrytis cinerea for each treatment group as recorded for 7 days following a post-inoculation treatment, where fungus was grown for 4 days prior to treatment and antifungal activity was determined (n = 24)
There was a significant difference in growth rate between treatment groups for C. herbarum killing (Kruskal-Wallis rank sum test; X2 = 226.25; DF = 3; P < 0.0001). The growth rates of product 102 and Green Earth were significantly different from the growth rate of both the positive and negative control groups (Table 2). The growth rates of product 102 and Green Earth were negative, indicating that the products kill the fungus (Fig. 4B).
There was a significant difference in growth rate between treatment groups for B. cinerea growth inhibition (Kruskal-Wallis rank sum test; X2 = 326.37; DF = 3; P < 0.0001). The growth rate of the positive control group was significantly higher from the growth rate of the negative control group, product 102, and Green Earth (Table 1; Fig. 4C).
There was a significant difference in growth rate among treatment groups for B. cinerea killing (Kruskal-Wallis rank sum test; X2 = 45.20; DF = 3; P < 0.0001). The growth rate of Green Earth was significantly different from the growth rate of the negative control group, positive control group, and product 102 (Table 2). Green Earth seemed to allow for fungal growth (Fig. 4D), indicating that Green Earth was not effective at killing B. cinerea. Product 102 was also not effective as a fungicide, but it was effective as fungistatic product.
Contact toxicity tests of product 102 on Bombus impatiens
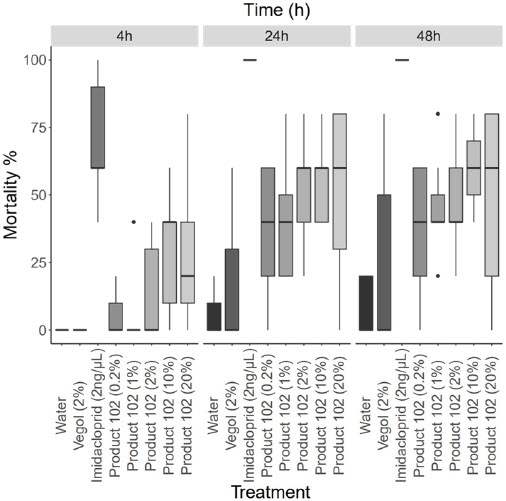
Concentration of product 102 significantly impacted mortality of bees at 4 h (glm; family = binomial; link = probit; Pr(>|z|) < 0.001) (Fig. 5). LD50 of product 102 at 4 h is 25.4% (V/v) ± 6.8%. There was not a significant impact of concentration of product 102 at 24 h (Fig. 5). LD50 of product 102 at 24 h is 13.7% (V/v) ± 5.6%. Concentration of product 102 significantly impacted mortality of bees at 48 h (glm; family = binomial; link = probit; Pr(>|z|) < 0.05) (Fig. 5). LD50 of product 102 at 48 h is 11.8% (V/v) ± 4.1%.
Time and concentration of product 102 significantly impacted bee mortality, where mortality increased over time and with increasing the concentration of product 102 (Fig. 5). All concentrations of product 102 have a significant impact on bee mortality (Table 3; Fig. 5). Imidacloprid significantly impacted bee mortality at all time intervals (Table 3; Fig. 5).
Figure 5
Mortality of Bombus impatiens Cresson from contact toxicity of several treatment groups, where 10 μL of solution was applied to the thorax
Mortality is shown for 4 h, 24 h, and 48 h after treatment (n = 25). The bar represents the median, the box represents the interquartile range, and the whiskers represent the maximum and minimum values that are not outliers.
Table 3
Fixed effects of linear mixed model fit by maximum likelihood, where t-tests use Satterthwaite’s method (“lmerModLmerTest”), for bee mortality data at 4 h, 24 h and 48 h, where n = 25
Product 102 was tested at concentrations 0.2% v/v, 1% v/v, 2% v/v, 10% v/v and 20% v/v.
Significant codes: *** = P < 0.001, ** = P < 0.01, * = P < 0.05, . = P < 0.1.
discussion
We found that product 102 has fungicidal activities, as well as exhibited a low contact toxicity to B. impatiens. The results of the topical and residual toxicity tests do not demonstrate any significant acaricidal effect of product 101 or 102 (essential oil-based products) on mites. However, previous studies conducted in our lab demonstrate the repellent effect of product 102 on T. urticae (data not shown).
Interestingly, the results of our study indicate that C. sativa is not a suitable host plant to T. urticae. A study done by Górski et al. (2016) examined the residual toxic effects of hemp essential oil against T. urticae when treating bean plants with the oil. Górski et al. (2016) determined that the essential oil produced from hemp has a significant effect on T. urticae mortality, where the strongest effect was observed when testing at the highest concentration of 0.1% (v/v). Additionally, hemp essential oil has shown repellent properties against the cabbage butterfly (Pieris brassicae L.) and the Japanese beetle (Popillia japonica Newman) (MacPortland 1997; Rothschild and Fairbairn 1980). Secondary metabolites, predominantly cannabinoids and terpenes, play an important role in the plant’s defence system, which can be triggered by biotic or abiotic stresses. When infestations of T. urticae feed on C. sativa, this can trigger cannabinoids and terpenes to be secreted by the trichomes of the plant (Kostanda and Khatib 2022). Future studies should investigate the acaricidal and repellent properties of Cannabis essential oil for the mana-gement of T. urticae. Although there is some support that C. sativa exhibits acaricidal properties to T. urticae, including our study, T. urticae does feed on C. sativa and causes significant plant damage (Park and Lee 2002). A possible explanation of how T. urticae feeds on C. sativa while being susceptible to the plant and/or essential oil is that T. urticae might develop a host plant adaptation to Cannabis over time (Hu et al. 2022). Intraspecific genetic variation in host preference has been shown in multiple studies for T. urticae, as is true of many other polyphagous pests (Fry 1989; Futuyma and Peterson 1985; Gould 1979; Via 1990). When the species encounters a new host plant and/or a plant treated with an acaricide, it could rapidly select for a genotype carrying a set of genes whose expression best buffer against the chemicals of the new hostile environment (Dermauw et al. 2013). Dermauw et al. (2013) provided an explanation for T. urticae’s ability to evolve with C. sativa. Additionally, T. urticae’s poly-phagy indicates an outstanding ability to adapt its digestive physiology and to overcome a wide range of defensive chemicals produced by host plants (Bensoussan et al. 2018).
Product 102 was determined to be effective at inhibiting growth and killing C. herbarum, which is one of the most common fungi isolated from the environment. A review completed by Whiley et al. (2018) investigated the antifungal properties of a variety of essential oils against common indoor fungal species. The review found that clove oil, tea tree oil, oregano, thyme, and lemon essential oils are all effective antifungal agents against a large number of fungi isolated from indoor environments. Clove oil has been most extensively researched and has demonstrated as an effective fungicide against common species such as Aspergillus versicolor, A. niger, A. fumigatus, Cladosporium sphaerospermum, C. cladosporioides, Penicillium chrysogenum, P. aurantiogriseum, P. digitatum, P. simplicissimum, and Ulocladium chartarum (Levinskaitė and Paškevičius 2013). Our study found similar findings where product 102 (essential oil-based) was effective at inhibiting growth and killing C. herbarum.
Product 102 was found to be effective at preventing the growth of B. cinerea, but it was not effective at killing the fungus once present. Bouchra et al. (2003) tested the essential oils of Origanum compactum L. and Thymus glandulosus L. against B. cinerea. The study determined that the mycelium growth of B. cinerea was completely inhibited at the concentration of 100 ppm. The inhibitory effect was mainly associated with the two most abundant components, thymol and carvacrol. Several other authors have also studied the antimicrobial activity of thymol and carvacrol (Arras and Usai 2001; Curtis et al. 1996; Kim et al. 1995).
There is limited knowledge regarding the antifungal mechanism of action of essential oils (Singh and Chittenden 2010; Verma et al. 2011). The active components of essential oils are mainly phenols, terpenes, aldehydes, and ketones (Ceylan and Fung 2004) and it is generally concluded that these functional groups act against cell membranes of microorganisms. That many essential oils are hydrophobic contributes to their ability to accumulate in the cell membrane, which disrupts cell structure and increases cell permeability (Lv et al. 2011). The mode of action of most antifungal compounds, including those antifungal essential oils which have been studied, is based on targeting either the formation or the function of ergosterol, an important component of the fungal cell membrane (Hector 1993; Pinto et al. 2009). This membrane interaction weakens the cell structure and increases permeability, which causes cell leakage and eventually cell lysis. Shao et al. (2013) determined that this is the mechanism of action for tea tree oil preventing the growth of B. cinerea, where the cell wall structure of B. cinerea was reported to have lost its ultrastructure and showed rupturing. This could be a putative mode of action for product 102 against C. herbarum and B. cinerea.
Whiley et al. (2018) noted that there are limited studies that scale up laboratory results and assess the efficacy of essential oils indoors. One of the leading indoor air quality concerns is the presence of fungi, which have been associated with increased risk of adverse health effects such as respiratory conditions and allergies (Arthur, 2005; Bird et al. 2012; Nevalainen et al. 2015). Future studies could focus on larger scale testing (within home and commercial buildings) of essential oils against common indoor fungi species, including realistic application methods and the potential for long-term antifungal persistence (Whiley et al. 2018).
We determined that the LD50 for B. impatiens of product 102 for 4, 24 and 48 h is 25.4%, 13.7%, and 11.8% (v/v) respectively. Given that the recommended concentration for product 102 application is 1:50 (2% v/v) the results of our study suggest that product 102 is unlikely to cause mortality to B. impatiens when applied at the recommended concentration within greenhouses or residential areas. Additionally, product 102 is significantly less toxic to B. impatiens compared to synthetic pesticides such as imidacloprid (average LD50 of 2 ng μL-1) (Marletto et al. 2003). Matos et al. (2021) compared the toxicity of synthetic insecticides deltamethrin and imidacloprid to the essential oil of Lippia sidoides Cham (rosemary pepper) against the stingless bee Nannotrigona aff. Testaceicornis Lepeletier (Hymenoptera: Apidae). The study determined that L. sidoides essential oil and its major component thymol have low lethal and sublethal toxicity to N. aff. Testaceicornis. Our study suggests the use of less hazardous alternatives to neurotoxic pesticides to protect our pollinators.
Overall, product 102 is effective at preventing the growth of two known fungal species of economic and health concern (C. herbarum and B. cinerea). Additionally, the product is significantly less toxic to B. impatiens when compared to a frequently used neonicotinoid insecticide. Our study provides support for the use of essential oil-based products for fungal management, as well as a safe alternative to synthetic insecticides for protecting pollinators.
Parties annexes
Acknowledgements
We would like to acknowledge that this study was conducted in Mi’kma’ki, the ancestral and unceded territory of the Mi’kmaq. We would like to thank Nutrilife Plant Products Limited for supporting our research and providing materials. We would like to thank the K.C. Irving Environmental Science Centre for the use of growth chambers, phytotrons and research space. We would like to thank Sarah Adams and Dr. Allison Walker for their assistance and advice when culturing species of fungi. Additionally, we would like to thank Wendy Hillier, Luca Voscort, Sarah Hobbs, and Tyler Peskett for their assistance with data collection.
References
- Arras, G., and M. Usai. 2001. Fungitoxic activity of 12 essential oils against four postharvest citrus pathogens: chemical analysis of thymus capitatus oil and its effect in subatmospheric pressure conditions. J. Food Prot. 64: 1025-1029.
- Arthur, R. 2005. Damp indoor spaces and health. Institute of Medicine: Committee on Damp Indoor Spaces and Health. The National Academy Press. ISBN 0-309-09193-4. Washington, D.C. 2004, pp. 355. J. Public Health 27: 234.
- Bakkali, F., S. Averbeck, D. Averbeck, and M. Idaomar. 2008. Biological effects of essential oils – a review. Food Chem. Toxicol. 46: 446-475.
- Bensoussan, N., V. Zhurov, S. Yamakawa, C.H. O’Neil, T. Suzuki, M. Grbić, and V. Grbić. 2018. The digestive system of the two-spotted spider mite, Tetranychus urticae Koch, in the context of the mite-plant interaction. Front. Plant Sci. 9: 1206.
- Bird, C., S. Balshaw, and W. Anderson. 2012. Getting the best answer by asking the right question – case studies in occupational exposure to mould. J. Health Saf. Res. Pract. 4: 19-27.
- Bonmatin, J.-M., C. Giorio, V. Girolami, D. Goulson, D.P. Kreutzweiser, C. Krupke, M. Liess, E. Long, M. Marzaro, E.A.D. Mitchell, D.A. Noome, N. Simon-Delso, and A. Tapparo. 2015. Environmental fate and exposure; neoni-cotinoids and fipronil. Environ. Sci. Pollut. Res. 22: 35-67.
- Bouchra, C., M. Achouri, L.M. Idrissi Hassani, and M. Hmamouchi. 2003. Chemical composition and antifungal activity of essential oils of seven Moroccan Labiatae against Botrytis cinerea Pers: Fr. J. Ethnopharmacol. 89: 165-169. doi:10.1016/S0378-8741(03)00275-7
- Carvalho, D.D.C., S.C.M. de Mello, M. Lobo Júnior, and A.M. Geraldine. 2011. Biocontrol of seed pathogens and growth promotion of common bean seedlings by Trichoderma harzianum. Pesq. Agropec. Bras. 46: 822-828. doi:10.159 0/S0100-204X2011000800006
- Ceylan, E., and D.Y.C. Fung. 2004. Antimicrobial activity of spices. J. Rapid Methods Autom. Microbiol. 12: 1-55.
- Codling, G., Y. Al Naggar, J.P. Giesy, and A.J. Robertson. 2016. Concentrations of neonicotinoid insecticides in honey, pollen and honey bees (Apis mellifera L.) in central Saskatchewan, Canada. Chemosphere 144: 2321–2328.
- Curtis, O.F., K. Shetty, G. Cassagnol, and M. Peleg. 1996. Comparison of the inhibitory and lethal effects of synthetic versions of plant metabolites (anethole, carvacrol, eugenol, and thymol) on a food spoilage yeast (Debaromyces hansenii). Food Biotechnol. 10: 55-73.
- Dermauw, W., N. Wybouw, S. Roumbauts, B. Menten, J. Vontas, M. Grbić, R.M. Clark, R. Feyereisen, and T. Van Leeuwen. 2013. A link between host plant adaptation and pesticide resistance in the polyphagous spider mite Tetranychus urticae. Proc. Natl. Acad. Sci 110: E113-E122.
- Dhingra, O.D., C.B. Maia, D.C. Lustosa, and J.B. Mesquita. 2002. Seedborne pathogenic fungi that affect seedling quality of red angico (Anadenanthera macrocarpa) trees in Brazil. J. Phytopathol. 150: 451-455.
- Elmer, P.A.G., and T. Reglinski. 2006. Biosuppression of Botrytis cinerea in grapes. Plant Pathol. 55: 155-177.
- Fairbrother, A., J. Purdy, T. Anderson, and R. Fell. 2014. Risks of neonicotinoid insecticides to honeybees. Environ. Toxicol. Chem. 33: 719-731. doi:10.1002%2Fetc.2527
- Fierascu, R.C., I.C. Fierascu, C.E. Dinu-Pirvu, I. Fierascu, and A. Paunescu. 2020. The application of essential oils as a next-generation of pesticides: recent developments and future perspectives. Z. Naturforsch. C 75: 183-204.
- Fry, J.D. 1989. Evolutionary adaptation to host plants in a labo-ratory population of the phytophagous mite Tetranychus urticae Koch. Oecologia 81: 559-565.
- Futuyma, D.J., and S.C. Peterson. 1985. Genetic variation in the use of resources by insects. Annu. Rev. Entomol. 30: 217-238.
- Gonzalez, M.F., F. Magdama, L. Galarza, D. Sosa, and C. Romero. 2020. Evaluation of the sensitivity and synergistic effect of Trichoderma reesei and mancozeb to inhibit under in vitro conditions the growth of Fusarium oxysporum. Commun. Integr. Biol. 13: 160-169.
- Górski, R., K. Sobieralski, and M. Siwulski. 2016. The effect of hemp essential oil on mortality Aulacorthum solani Kalt. And Tetranychus urticae Koch. Ecol. Chem. Eng. S 23: 505-511.
- Gould, F. 1979. Rapid host range evolution in a population of the phytophagous mite Tetranychus urticae Koch. Evolution 33: 791-802. doi:10.2307/2407646
- Grbic, M., A. Khila, K.-Z. Lee, A. Bjelica, V. Grbic, J. Whistlecraft, L. Verdon, M. Navajas, and L. Nagy. 2007. Mity model: Tetranychus urticae, a candidate for chelicerate model organism. BioEssays 29: 489-496.
- He, L., Y. Liu, A. Mustapha, and M. Lin. 2011. Antifungal activity of zinc oxide nanoparticles against Botrytis cinerea and Penicillium expansum. Microbiol. Res. 166: 207-215.
- Hector, R.F. 1993. Compounds active against cell walls of medically important fungi. Clin. Microbiol. Rev. 6: 1-21. doi:10.1128%2Fcmr.6.1.1
- Hu, Q.-Q., X.-Y. Yu, X.-F. Xue, X.-Y. Hong, J.-P. Zhang, and J.-T.Sun. 2022. Phylogenetic-related divergence in perceiving suitable host plants among five spider mites species (Acari: Tetranychidae). Insects 13: 705.
- Isman, M.B. 2000. Plant essential oils for pest and disease management. Crop Prot. 19: 603-608. doi:10.1016/S0261-2194(00)00079-X
- Isman, M.B. 2006. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol. 51: 45-66.
- Isman, M.B., and C.M. Machial. 2006. Pesticides based on plant essential oils: from traditional practice to comer-cialization. Pages 29-44 in M. Rai and M.C. Carpinella (eds.), Advances in phytomedicine, Elsevier. doi:10.1016/S1572-557X(06)03002-9
- Kim, J., M.R. Marshall, and C.-I Wei. 1995. Antibacterial activity of some essential oil components against five foodborne pathogens. J. Agric. Food Chem. 43: 2839-2845.
- Klein, A.-M., B.E. Vaissière, J.H. Cane, I. Steffan-Dewenter, S.A. Cunningham, C. Kremen, and T. Tscharntke. 2006. Importance of pollinators in changing landscapes for world crops. Proc. Royal Soc. B 274: 303-313.
- Kostanda, E., and S. Khatib. 2022. Biotic stress caused by Tetranychus urticae mites elevates the quantity of secondary metabolites, cannabinoids and terpenes, in Cannabis sativa L. Ind. Crops Prod. 176: 114331.
- Leroux, P. 2007. Chemical control of Botrytis and its resistance to chemical fungicides. Pages 195-222 in Y. Elad, B. Williamson, P. Tudzynski, and N. Delen (eds.), Botrytis: biology, pathology and control. Springer, Dordrecht, Netherlands.
- Levinskaitė, L., and A. Paškevičius. 2013. Fungi in water-damaged buildings of Vilnius old city and their suscep-tibility towards disinfectants and essential oils. Indoor Built Environ. 22: 766-775.
- Lv, F., H. Liang, Q. Yuan, and C. Li. 2011. In vitro antimicrobial effects and mechanism of action of selected plant essential oil combinations against four food-related microorganisms. Food Res. Int. 44: 3057-3064.
- MacPortland, J.M. 1997. Cannabis as repellent and pesticide. J. Int. Hemp Assoc. 4: 87-92.
- Marletto, F., A. Patetta, and A. Manino. 2003. Laboratory assessment of pesticide toxicity to bumblebees. Bull. Insectology 56: 155-158.
- Matos, W.B., A.C.C. Santos, A.P.S. Lima, E.D.R. Santana, J.E. Silva, A.F. Blank, A.P.A. Araújo, and L. Bacci. 2021. Potential source of ecofriendly insecticides: essential oil induces avoidance and cause lower impairment on the activity of a stingless bee than organosynthetic insecticides, in laboratory. Ecotoxicol. Environ. Saf. 209: 111764.
- Medrzycki, P., H. Giffard, P. Aupinel, L.P. Belzunces, M.-P. Chauzat, C. Claßen, M.E. Colin, T. Dupont, V. Girolami, R. Johnson, Y. Le Conte, J. Lückmann, M. Marzaro, J. Pistorius, C. Porrini, A. Schur, F. Sgolastra, N. Simon-Delso, J.J.M. van der Steen, K. Wallner, C. Alaux, D.G. Biron, N. Blot, G. Bogo, J.-L. Brunet, F. Delbac, M. Diogon, H. El Alaoui, B. Provost, S. Tosi, and C. Vidau. 2013. Standard methods for toxicology research in Apis mellifera. J. Apic. Res. 52: 1-60.
- Nevalainen, A., M. Täubel, and A. Hyvärinen. 2015. Indoor fungi: companions and contaminants. Indoor Air 25: 125-156.
- Park, Y.-L., and J.-H. Lee. 2002. Leaf cell and tissue damage of cucumber caused by two spotted spider mite (Acari: Tetranychidae). J. Econ. Entomol. 95: 952-957.
- Pinto, E., L. Vale-Silva, C. Cavaleiro, and L. Salgueiro. 2009. Antifungal activity of the clove essential oil from Syzygium aromaticum on Candida, Aspergillus and dermatophyte species. J. Med. Microbiol. 58: 1454-1462.
- Raveau, R., J. Fontaine, and A. Lounès-Hadj Sahraoui. 2020. Essential oils as potential alternative biocontrol products against plant pathogens and weeds: a review. Foods 9: 365.
- Regnault-Roger, C., C. Vincent, and J.T. Arnason. 2012. Essential oils in insect control: low-risk products in a high-stakes world. Annu. Rev. Entomol. 57: 405-424.
- Rothschild, M., and J.W. Fairbairn. 1980. Ovipositing butterfly (Pieris brassicae L.) distinguishes between aqueous extracts of two strains of Cannabis sativa L. and THC and CBD. Nature 286: 56-59.
- Samson, R.A., E.S. Hoekstra, J.C. Frisvad, and O. Filtenborg. 2000. Introduction to food- and airborne fungi. Centraal-bureau voor Schimmelcultures, Utrecht, Netherlands. 389 pp.
- Shao, X., S. Cheng, H. Wang, D. Yu, and C. Mungai. 2013. The possible mechanism of antifungal action of tea tree oil on Botrytis cinerea. J. Appl. Microbiol. 114: 1642-1649.
- Singh, T., and C. Chittenden. 2010. Efficacy of essential oil extracts in inhibiting mould growth on panel products. Build. Environ. 45: 2336-2342.
- Tripathi, A.K., S. Upadhyay, M. Bhuiyan, and P.R. Bhattacharya. 2009. A review on prospects of essential oils as biopesticide in insect-pest management. J. Pharmacogn. Phytotherapy 1: 52-63.
- Tripathi, P., N.K. Dubey, and A.K. Shukla. 2008. Use of some essential oils as post-harvest botanical fungicides in the management of grey mould of grapes caused by Botrytis cinerea. World J. Microbiol. Biotechnol. 24: 39-46.
- Verma, R.K., L. Chaurasia, and M. Kumar. 2011. Antifungal activity of essential oils against selected building fungi. Indian J. Nat. Prod. Resour. 2: 448-451.
- Via, S. 1990. Ecological genetics and host adaptation in herbivorous insects: the experimental study of evolution in natural and agricultural systems. Annu. Rev. Entomol. 35: 421-446.
- Whiley, H., S. Gaskin, T. Schroder, and K. Ross. 2018. Anti-fungal properties of essential oils for improvement of indoor air quality: a review. Rev. Environ. Health 33: 63-76.
- Xu, D., Y. He, Y. Zhang, W. Xie, Q. Wu, and S. Wang. 2018. Status of pesticide resistance and associated mutations in the two-spotted spider mite, Tetranychus urticae, in China. Pestic. Biochem. Physiol. 150: 89-96.
- Yamada, T., K. Yamada, and N. Wada. 2012. Influence of dinotefuran and clothianidin on a bee colony. Jpn. J. Clin. Oncol. 21: 10-23.
Liste des figures
Figure 1
Mortality of Tetranychus urticae following topical treatment of Nutrilife product 101 and 102, control (water), and Vegol® Crop Oil on a variety of plant species (cucumber, hemp, hops, kale, lettuce, tomato), where mortality was recorded at 1hr, 2 h, 4 h, and 24 h post-treatment
Figure 2
Mortality of Tetranychus urticae following topical treatment of Nutrilife product 101 and 102, control (water), and Vegol® Crop Oil on hemp (Cannabis sativa), where mortality was recorded at 1 h, 2 h, 4 h, and 24 h post-treatment (n = 25)
Figure 3
Mortality of Tetranychus urticae following residual treatment of Nutrilife product 101 and 102, control (water), and Vegol® Crop Oil on a variety of plant species (bean, hemp, and hops), where mites were transferred at 2 h, 24 h, 48 h, and 72 h post-treatment
Figure 4
Growth rate of Cladosporium herbarum and Botrytis cinerea for each treatment group as recorded for 7 days following a pre- or post-inoculation treatment, where prevention of fungus growth or killing of fungus was determined (n = 24)
(A): The prevention of Cladosporium herbarum growth. (B): The killing of Cladosporium herbarum. (C): The prevention of Botrytis cinerea growth. (D): The killing of Botrytis cinerea. The bar represents the median, the box represents the interquartile range, and the whiskers represent the maximum and minimum values that are not outliers.
Figure 5
Mortality of Bombus impatiens Cresson from contact toxicity of several treatment groups, where 10 μL of solution was applied to the thorax
Liste des tableaux
Table 1
Dunn multiple comparison with the Bonferroni method for growth rate of Cladosporium herbarum and Botrytis cinerea for each treatment group as recorded for 7 days following a pre-inoculation treatment, where prevention of fungal growth was determined (n = 24)
Table 2
Dunn multiple comparison with the Bonferroni method for growth rate of Cladosporium herbarum and Botrytis cinerea for each treatment group as recorded for 7 days following a post-inoculation treatment, where fungus was grown for 4 days prior to treatment and antifungal activity was determined (n = 24)
Table 3
Fixed effects of linear mixed model fit by maximum likelihood, where t-tests use Satterthwaite’s method (“lmerModLmerTest”), for bee mortality data at 4 h, 24 h and 48 h, where n = 25