Résumés
Abstract
The aim of this work was to develop new low-cost adsorbents obtained from animal origins, available in large quantities and environmentally friendly. Raw shrimp shell (RSS), a biomaterial of animal origin, is abundant, available, renewable and non-toxic. It has physicochemical properties that can induce a significant adsorptive activity. In this study, the removal of phosphate anions (H2PO4-, HPO42-) from aqueous solution by adsorption onto raw shrimp shells was studied. The surface micro-morphology of the biomaterial was investigated using scanning electron microscope and qualitative element composition was analyzed using energy dispersive X-ray and infrared spectroscopies. The efficiency of the biomaterial was investigated using a batch adsorption technique under different experiment conditions, achieved by varying parameters such as adsorbent dosage, the contact time, the initial phosphate anion concentrations, the temperature and the initial solution pH. Results show that the kinetics adsorption of phosphate ions by the biomaterial is relatively quick and the biomaterial showed a high adsorption capacity of 0.20 g∙g-1 and 0.4 g∙g-1 for HPO42- and H2PO4-, respectively. The adsorption data were analyzed using the Langmuir, Freundlich and Temkin adsorption isotherms to determine the nature of the adsorption sites. Both Langmuir and Freundlich adsorption models showed good fits to the experimental adsorption data.
Keywords:
- Adsorption,
- orthophosphate,
- shrimp shell,
- wastewater
Résumé
Cette étude s’inscrit dans le cadre de certains de nos travaux visant la valorisation de matériaux naturels d’origine végétale, animale et minérale pouvant rendre les procédés d’épuration des eaux simples et moins coûteux. Les carapaces de crevettes à l’état brut, biomatériau d’origine animale, entrent dans cette catégorie. Il est abondant, disponible, renouvelable et non toxique, et présente des propriétés physicochimiques qui peuvent induire une activité adsorbante importante. Le biomatériau a été caractérisé par microscopie électronique à balayage, par spectroscopie d’émission X et spectroscopie infrarouge. Une étude complète de l’adsorption des ions phosphates (HPO42- et H2PO4-) sur des carapace de crevettes à l’état brut a été effectuée. L’influence de paramètres physicochimiques tels que la masse d’adsorbant, le temps de contact, le pH, la température et la concentration initiale de phosphate a été étudiée. Les résultats obtenus montrent une cinétique rapide et une grande capacité de ce biomatériau à retenir les ions phosphates pouvant atteindre 0,20 g∙g-1 pour HPO42- et 0,40 g∙g-1 pour H2PO4-. Les isothermes d’adsorption étudiés (Langmuir, Freundlich et Temkin) montrent une bonne corrélation avec les modèles de Langmuir et de Freundlich.
Mots-clés :
- Adsorption,
- orthophosphates,
- carapace de crevette,
- eaux usées
Corps de l’article
1. Introduction
Phosphorus is the key nutrients for biological and chemical processes in natural water bodies, and large quantity of phosphate present in wastewater is one of the main causes of eutrophication in surface waters (CHIBAN et al., 2012; RODIER, 2009; NGUYEN et al., 2012; VIESSMAN and HAMMER, 2005). Morocco has very limited water resources. Besides drought cycles, the already scarce water is the subject of the continuous increasing needs and growing quality degradation due to pollution. The nature of the pollution charge of quality degradation is mainly organic type, nitrogen and phosphorus. The eutrophication of rivers and dams deductions was observed; oxygen levels in some reservoirs have fallen and have promoted specific anaerobic conditions. In terms of phosphorus, the values stored in some dams have exceeded 0.5 mg∙L-1 (OBSERVATOIRE NATIONAL DE L'ENVIRONNEMENT ET DU DÉVELOPPEMENT DURABLE DU MAROC, 2015).
Various techniques have been used for phosphate and heavy metals removal. Phosphate removal techniques fall into three main categories: physical, chemical and biological. The major disadvantage that we come across with the conventional processes is that the process is expensive and not eco-friendly (CHIBAN et al., 2013; NGUYEN et al., 2012; WAN NGAH and HANAFIAH, 2008). Though in the recent years the focus of the research is to use biocompatible materials as potential adsorbents. This solution can prove unpollutant, economic and able to bring cost effectiveness. Research based on animal, vegetal and inorganic adsorption materials, have been used by many researchers (CHUBAR et al., 2005; DE LIMA et al., 2012; DIVYA JYOTHI et al., 2012; ISMAIL, 2012; KARACA et al., 2006; LI et al., 2006; NGUYEN et al., 2012; SAAD et al., 2007; WAN NGAH and HANAFIAH, 2008; YOUCEF and ACHOUR, 2005; ZHANG et al., 2010). An adsorbent can be considered as cheap or low-cost if it is abundant in nature, requires little processing and is a byproduct of waste material from waste industry (BAILEY et al., 1999). The raw shrimp shells, is a residue of animal origin, easily available and inexpensive, seems to be a suitable material for phosphate sorption. Actually chitin is one of the most abundant biopolymers in nature and is a major component of shrimp shells. Several studies have clearly demonstrated that chitin (and its deacetylated derivative, chitosan), have gained wide attention as effective biosorbents due to low cost and high contents of amino and hydroxyl functional groups which show significant adsorption potential for the removal of various aquatic pollutants (BHATNAGAR and SILLANPÄÄ, 2009). Moreover, the use of these shells as raw material for wastewater treatment could also increase the additional value of this biomass waste that could potentially become an environmental problem.
The present work was performed to evaluate the capacity of adsorption of the raw shrimp shells, for removing phosphate anions (HPO42- and H2PO4-) from aqueous solutions. The study of the influence of various parameters such as adsorbent dosage, contact time, pH, temperature and initial adsorbate concentration on adsorption were investigated.
2. Materials and methods
2.1 Materials
The inert solid biomaterial (ISBM) used as support adsorption in this work is the raw shrimp shells (RSS). The shrimp shells were washed thoroughly with pure water several times. The washed materials were then air-dried (≈27 °C), grinded and sieved (≤500 µm). The microparticles of the raw shrimp shells are used as adsorbent materials in batch experiments without any pretreatment to avoid extra expenditure.
Crustacean shell waste consists mainly of 30-40% protein, 30-50% calcium-carbonate, and 20-30% chitin (JOHNSON and PENISTON, 1982; KURITA, 2006). Crab shells consist of CaCO3 and chitin, usually cross-linked with some protein, and a proportion of lipids (Figure 1) (JOHNSON and PENISTON, 1982; KURITA, 2006).
Figure 1
Schematization of the raw shrimp shells (JOHNSON and PENISTON, 1982; KURITA, 2006)
Schématisation de carapaces de crevettes à l’état brut (JOHNSON et PENISTON, 1982; KURITA, 2006)
The point of zero charge (PZC) was determined using the solid addition method (BANERJEE and CHATTOPADHYAYA, 2013) viz: 50 mL of 0.01 M NaCl solutions were taken in different conical flasks of 100 mL and 0.5 g of adsorbent was introduced in each of them. Now pH values of these solutions were adjusted in 2 to 12 range by 0.1 M HCl/NaOH solutions. These flasks were kept for 48 h and the final pH of the solutions was measured. Graphs were plotted between pHfinal versus pHinitial. The point of intersection of the curve of pHfinal versus pHinitial was recorded as pHPZC of the shrimp shells.
2.2 Solutions of the studied ions
NaH2PO4 and Na2HPO4∙12H2O salts were analytical grade reagents from Fluka. Aqueous solutions of these salts were prepared in de-ionized water. The concentration range of the solution ion prepared varies between 50 mg∙L-1 and 7 g∙L-1. For the influence of PH in the adsorption experiments, the pH of the solution was adjusted by adding dilute solution of hydrochloric acid (1 M HCl) or sodium hydroxide (1 M NaOH).
2.3 Batch adsorption studies
Batch adsorption experiments were carried out by batch process. 40 ml of phosphate solution with a given ions concentration (Ci) (mg∙L-1), was mixed with 1 g of dried and grinded shrimp shells. The solutions put in contact with the raw shrimp shells matter were maintained at a constant temperature of 25 °C in a water bath thermostat, the mixture being vigorously stirred at 180 rpm. The sampled solutions were then centrifuged at 500 rpm and the concentration of phosphate anions present in the liquid phase (supernatant) was determined using UV spectrophotometer. The mass/volume ratio, used in this work, must correspond to the smallest weight of ISBM giving the highest uptake percentage of phosphate.
2.4 Analytical procedure and method for calculating Cr (mg∙L-1) and Qads (mg∙g-1)
After all the kinetic and equilibrium studies, the resulting solution was filtered by Whatman filter paper (0.45 μm) and the filtrate was analyzed. The phosphate anions concentrations was determined by the formation of ammonia phosphomolybdate and the subsequent reduction with ascorbic acid, followed by spectrophotometric measurements at 700 nm, using the spectrophotometric method (AFNOR T 90-023) (RODIER, 2009).
Each experiment was carried out in duplicate and the average results are presented in this study. The variation of the adsorbed phosphate concentration (Cr) (mg∙L-1) represented in the figures is defined as Cr = Ci - Ce for the solid-liquid ratio 1 g/40 mL (m/V = 25 g∙L-1). The amount of phosphate anions adsorbed at equilibrium per unit mass of biomaterial was determined according to the following equation:
where m is the weight of the raw shrimp shells (g), V is the total solution volume (L), Ci and Ce are the initial and equilibrium concentration of pollutant solution (mg∙L-1), respectively, and Qads is the amount adsorbed at equilibrium per unit mass of ISBM (mg∙g-1).
The removal percentage of pollutant from aqueous solution X (%) was calculated by using the following expression:
3. Results and discussion
3.1 Characterization of the adsorbent
3.1.1 Analysis by scanning electron microscope
The surface micromorphology of the raw shrimp shells was investigated using scanning electron microscope (SEM, HITACHI S-4500) (Figure 2). SEM images of the raw shrimp shells microparticles (Figure 2a) shows that the dried biomaterial is not homogeneous. The SEM images indicated also the presence of grains in the structure at different sizes and morphologies. Figure 2b shows the raw shrimp shells had a highly porous surface, indicating higher surface area. The morphology of this biomaterial can facilitate the adsorption of anions and metallic elements, due to the irregular surface.
Figure 2
Scanning electron microscope image of the raw shrimp shells (particle size < 500 μm)
Image par microscopie électronique à balayage (MEB) de la poudre du broyat de carapaces de crevettes à l’état brut (granulométrie < 500 µm)
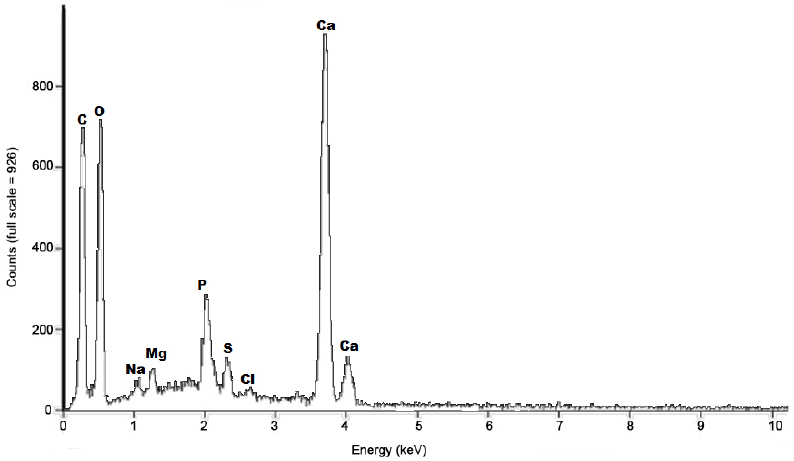
3.1.2 Chemical analysis by X-ray emission spectroscopy
The X-ray emission spectrum of the raw shrimp shells shows that in addition to carbon and oxygen; major constituents of proteins, chitin, and carbonates, are also composed largely of calcium (Figure 3) . This confirms that carbonates are mostly in the form of calcium bicarbonates as has been reported for crustacean shells (JOHNSON and PENISTON, 1982; KURITA, 2006; ROBERTS, 1992).
Figure 3
Energy dispersive X-ray spectrum of raw shrimp shell particles (particle size < 500 μm)
Spectre de rayons X à dispersion d'énergie de carapaces de crevettes à l’état brut (granulométrie < 500 µm)
3.1.3 Infrared spectroscopy
The Infrared spectrum (IR) of the raw shrimp shells, in KBr, (Figure 4) presents typical absorption bands found in the spectrum IR of the biomolecules (proteins, lipids, glucids and nucleic acids) (VAN HOLDE et al., 1998).
Figure 4
Infrared spectrum of the raw shrimp shells in KBr
Spectre infrarouge de carapaces de crevettes à l’état brut dans KBr
Hydroxyl groups (OH) and amine (NH) are represented by a broad band at 3 300 cm-1 and absorption of these groups in the region indicates that may be an attribution to the hydrogen bonds. Stretching vibration corresponds to C-H are also common to both spectra. They are characterized by absorption in the region of 2 800 cm-1 and 3 030 cm-1.
The absorption bands in the region of 1 500 and 1 650 cm-1 corresponds to carbonyl groups stretching vibrations. The absorption bands in the region of 1 030 cm-1 and 1 450 cm-1 corresponds to RC-H, R-OH and amines vibration. The broad band at 1 420 cm-1 is due to mineral salts, especially bicarbonates, characterized by CC, CO and CN groups.
3.2 Phosphate adsorption onto inert solid biomaterial (ISBM)
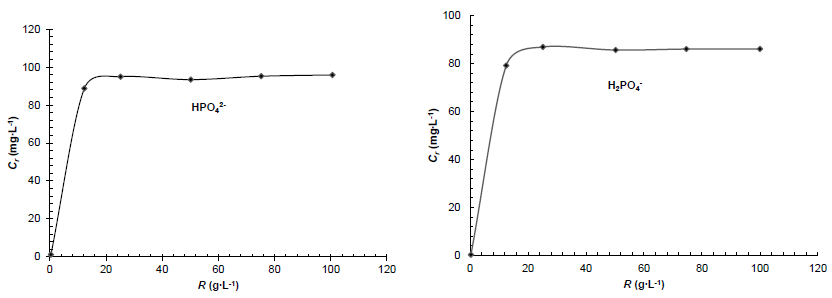
3.2.1 Determination of mass/volume ratio (R = m/V)
The ratio of the weight of ISBM adsorbent to volume of the aqueous phase is a very important parameter of the adsorption process. This ratio corresponding to the smallest weight of ISBM giving the highest uptake percentage of phosphate.
Different weight (m) of ISBM were shaken with V = 40 ml of phosphate solution of initial concentration value Ci = 100 mg∙L-1 for 24 h, the temperature of 25 °C and the pH of the solutions studied is imposed by the dissolved salt for each ion. Figure 5 shows the variation of the adsorbed phosphate anions concentration (Cr = Ci - Ce) as a function of the m/V ratio.
The results shown in Figure 5 for phosphate anions in contact with raw shell shrimp, indicated that the phosphate solution presented the highest uptake percentage up to the ratio value R = 25 g∙L-1 and any further addition of ISBM shows no significant increasing effect on the retention process. In order to achieve experiments for maximum retention of the phosphate anions, the m/V ratio has been chosen to be R = 25 g∙L-1 in all experiments.
Figure 5
Variation of the phosphate anion concentrations adsorbed by the raw shrimp shells as a function of the m/V ratio (R): Ci = 100 mg∙L-1, T = 25 °C and tc = 24 h
Variation de la concentration d'ions phosphates retenus par le broyat de carapaces de crevettes à l’état brut en fonction du rapport R (Ci = 100 mg∙L-1; T = 25 °C; tc = 24 h)
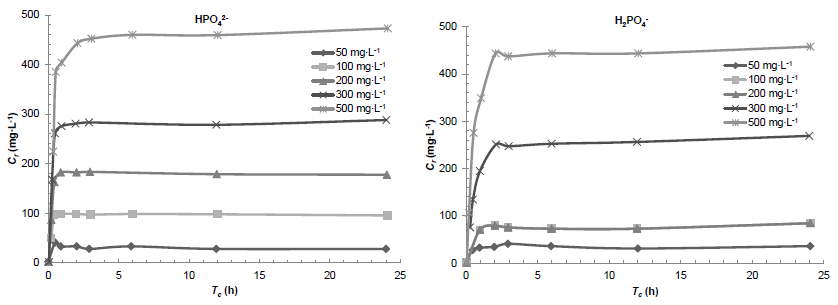
3.2.2 Effect of contact time
The dynamics of the phosphate anions uptake was studied in batch experiments by using an ISBM suspension of 25 g∙L-1 and an initial phosphate anions concentration of 50, 100, 200, 300, 500 mg∙L-1 (Figure 6), the averages pH are 7.6 and 5.1 imposed by the solutes used (NaH2PO4∙12H2O) and NaH2PO4 respectively.
Figure 6
Effect of contact time on the removal of the phosphate anions by the raw shrimp shells (R = 25 g∙L-1; pH = 7.55; T = 25 °C)
Variation de la concentration d’ions phosphates retenus par le broyat de carapaces de crevettes à l’état brut en fonction du temps de contact (R = 25 g∙L-1; pH = 7,55; T = 25 °C)
The variations of the concentrations of phosphates retained versus the contact time (tc) are plotted in Figure 6.
The process of phosphate uptake by the ISBM appeared to follow a two phase process characterized by an initial fast retention step in less than one hour, and corresponding to an uptake concentration of about 70-80% of the initial phosphate anions concentration of the solution, followed by a much slower step before steady state. Equilibrium (te) is reached in less than two hours.
Results of phosphate anions adsorption onto inert solid biomaterials of vegetal origin (SOUDANI et al., 2009) showed that the adsorption variation with contact time is composed of two regimes. In the first step, the ions located in the neighborhood of the surface of the particles of the crushed biomaterial are retained and an important percentage of the phosphate anions are removed. The second step is slower than the previous one and corresponds to the rate limiting step of the adsorption process. The diffusion of the ions towards the adsorption sites buried in the ISBM particles inner structure is presumably responsible for this slow adsorption regime.
The adsorbed amounts at equilibrium of HPO42- anions are little different than those of H2PO4- anions, total ionic charge do not have a very significant effect on the adsorption process. We can therefore conclude that the adsorption of phosphate anions is a chemical adsorption and involves other interactions in addition to the electrostatic interactions. This similarity between the adsorption results of both anions is valid for low and medium concentrations below 500 mg∙L-1, because as discussed in the following paragraph, this is not valid for high concentrations. At initial concentrations Ci < 500 mg∙L-1 we remain well below the total saturation of adsorption sites and therefore the impact of pH is not important. This is not valid for high concentrations as explained in the following paragraph relating to the determination of maximum adsorption capacity.
To investigate the adsorption mechanism and potential speed control steps, two kinetic models were applied: the pseudo-first order Lagergren kinetic model (LAGERGREN and SVENSKA, 1889) and the pseudo-second order kinetic model (HO and McKAY, 1998) were used. The pseudo-first order Lagergren model is expressed by:
where Qe (mg∙g-1) is the adsorbed amount of phosphate ions in equilibrium, Qt (mg∙g-1) adsorbed amount of phosphate ions at time t (min) and k1 (min-1) constant pseudo-first order rate.
Qe and k1 can be calculated from the slope and intercept of the log(Qe - Qt) versus t. Is checked, then, if the values of the adsorption capacity in balance calculated (Qe(cal)), are consistent with the experimental values of Qe (Qe(exp)) (OZACAR and SENGIL, 2003).
The kinetic model pseudo-second order is given by equation 4:
where k2 (g∙mg-1∙min-1) is constant pseudo-second order rate.
The adsorption capacity Qe at equilibrium and the constant of pseudo-second order rate k2 can be derived experimentally from the slope and intercept of the curve t/Qt as function of t.
The parameters of kinetic model pseudo-first and pseudo-second order related to the adsorption of phosphate anions on the shrimp shells are shown in Table 1.
Table 1
Parameters of pseudo-first order and pseudo-second order kinetic models for HPO42- and H2PO4- ions
Paramètres des modèles cinétiques du pseudo-premier ordre et pseudo-second ordre pour les ions HPO42- et H2PO4-
It is found that the adsorption of phosphate anions on shrimp shells is not a first order reaction. Although the values of the correlation coefficient (R2) are of the order of 0.99, the experimental values of Qe(exp) are not in agreement with those calculated Qe(cal).
For kinetic pseudo-second order model and as shown in Table 1, the good correlation (R2 = 0.99) is obtained for both initial concentrations (Ci = 100 mg∙L-1, Ci = 300 mg∙L-1 and Ci = 500 mg∙L-1) and reference value Qe(cal) are also in good agreement with the experimental values Qe(exp). This result suggests that the adsorption of phosphate anions on the shrimp shells is consistent with the pseudo-second order reaction mechanism and the adsorption rate is controlled by chemical adsorption (DOGAN et al., 2004).
3.2.3 Determination of maximum adsorption capacity by one gram of ISBM
In order to determine the maximum adsorption capacity by one gram of ISBM derived from grinded raw shrimp shells, effect of initial concentration on the removal of HPO42-, H2PO4- anions has been studied at varying initial concentration range between 0.5 g∙L-1 and 7 g∙L-1 (Figure 7). Initial pH range is between 7.81 and 9.28 for HPO42- solution, and between 5.20 and 6.75 for H2PO4- solution.
Figure 7
Effect of initial concentration on the removal of HPO42- and H2PO4- anions from single ion solutions by raw shrimp shells (R = 25 g∙L-1; T = 25 °C; tc = 6 h > te = 2 h)
Variation de la concentration d’ions phosphates retenus par le broyat de carapaces de crevettes à l’état brut en fonction de la concentration initiale (R = 25 g∙L-1; T = 25 °C; tc = 6 h > te = 2 h)
From these results obtained, the phosphate anions adsorbed concentrations increased with increasing initial concentration of these anions. Despite a high initial concentration equal to 7 g∙L-1 the saturation of crushed biomaterial is not reached.
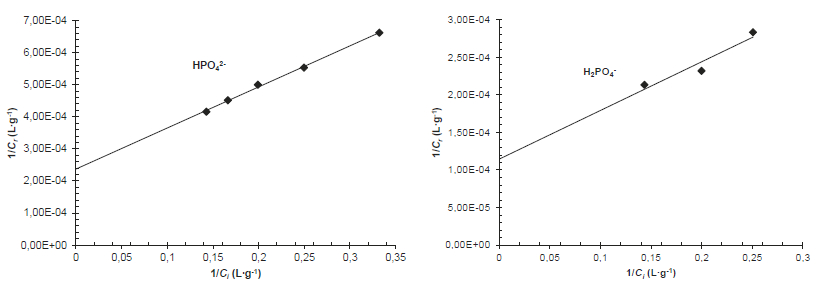
In order to determine the maximum amount adsorbed, the plots 1/Cr = f(1/Ci), for both phosphate anions HPO42- and H2PO4-, allow us to evaluate the limiting adsorption concentrations (Crmax), as shown in Figure 8.
Figure 8
Plot of inverse values of Cr vs. Ci corresponding to the data shown in Figure 7
Variation de 1/Cr en fonction de 1/Ci
The extrapolation to infinite concentration (Figure 8), the limiting adsorption concentrations for HPO42- and H2PO4- anions corresponds to 5.00 g∙L-1 and 10.00 g∙L-1, respectively 0.20 g∙g-1 and 0.40 g∙g-1 per unit mass of ISBM. These amounts are to much higher than those obtained with several other natural adsorbents (NGUYEN et al., 2012; SOUDANI et al., 2009; ISMAIL, 2012; BENYOUCEF and AMRANI, 2011; BISWAS et al., 2007; KRISHNAN and HARIDAS, 2008; MEZENNER and BENSMAILI, 2009). These results suggest that the raw shrimp shells have a significant potential adsorption towards the phosphate anions.
We also note that the maximum amount adsorbed for H2PO4- anions is higher than this of HPO42- anions. There is an increase in the difference between the pH of solutions of H2PO4- and HPO42- as initial concentration of these ions increases and pH of HPO42- becomes basic. This suggests that at high concentrations of Na2HPO4 salt, hydroxide ions compete with HPO42- ions for the exchange sites in the inert organic matter. That can explain the increase in HPO42- ions uptake in comparison with H2PO4-. Similar behavior was observed for other adsorbents of natural origin (ZHANG et al., 2010; CHIBAN, 2007; SOUDANI et al., 2009).
3.2.4 Adsorption isotherms
The adsorption isotherms are mathematical models that describe the distribution of the adsorbate species among solid and liquid phases, and are important data to understand the mechanism of the adsorption. Several models have been published in literature to describe the experimental data of the adsorption isotherms. Langmuir and Freundlich isotherms are the most frequently employed models. In this study, Langmuir, Freundlich and Temkin equations were used to describe the relationship between the adsorbed anion uptake on raw shell shrimp and its equilibrium concentration in solution. This study was carried out by varying the initial ion concentration from 0.5 g∙L-1 à 7 g∙L-1 at a temperature of 25 °C.
Langmuir isotherm (LANGMUIR, 1916):
where Ce is the equilibrium concentration (mg∙L-1), K the constant related to the affinity of the binding sites and energy of adsorption (L∙mg-1). A plot of 1/Cr versus 1/Ce should indicate a straight line of slope 1/KCrmax and an intercept of 1/Crmax. So, Qmax and K can be determined.
Freundlich isotherm (FREUNDLICH, 1906):
where Ce is the equilibrium concentration (mg∙L-1), Qads the amount of ion adsorbed (mg∙g-1), Kf and nf the Freundlich constants.
Temkin isotherm (TEMKIN and PYZHEV, 1940):
where K = f(g). The plots corresponding to the Langmuir, Freundlich and Temkin isotherms for HPO42- anions are illustrated in Figure 9.
Figure 9
Isotherm plots for the adsorption of HPO42- anions by the raw shrimp shells
Isothermes d'adsorption de HPO42- sur le broyat de carapaces de crevettes à l’état brut
a) Langmuir isotherm; b) Freundlich isotherm; c) Temkin isotherm
a) isotherme de Langmuir; b) isotherme de Freundlich; c) isotherme de Temkin
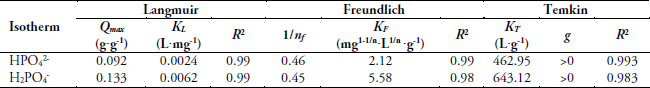
The correlation coefficients from Langmuir, Freundlich and Temkin isotherms are given in Table 2. The adsorption data in respect to HPO42- anions provide a good fit to Langmuir isotherm, based on the independence of adsorption sites and consequently the absence of interactions between the adsorbed ions. A good correlation is obtained also with the Freundlich isotherm that suggests the existence of several kinds of adsorption sites and reinforces the good correlation obtained with the Langmuir isotherm.
Table 2
Isotherm model parameters and correlation coefficients for HPO42- and H2PO4- ions
Paramètres et valeurs des coefficients de corrélation des isothermes pour les ions HPO42- et H2PO4-
Good correlation obtained with Langmuir isotherm can be explained by the presence of several constituents inside the ISBM responsible of the phosphate adsorption, such as chitin and proteins. The factor g given by Temkin isotherm is positive, as the sign of the slope of the isotherm (logarithimic form) is positive; the interactions involved are so repulsive and weak. This confirms the good correlation of Langmuir that neglects interactions between adsorbed species.
The isotherm constant 1/nf is less than 1 (1/nf < 1) suggesting that the adsorption sites are not homogeneous (CHIBAN, 2007). The low value of the correlation coefficients from Temkin model of H2PO4- (R2 = 0.9835) than HPO42- (R2 = 0.9933), can be related to the difference of the ionic charge of both anions.
Recall that the results of the adsorption of both anions at medium concentrations below 500 mg∙L-1 are slightly different (Figure 6 and Table 1), that suggest a low involvement of electrostatic interactions.
3.2.5 Effect of initial pH
In aqueous solution, phosphate anions exist in four forms. In strongly basic conditions, the phosphate ion (PO43−) predominates, whereas in weakly basic conditions, the hydrogen phosphate ion (HPO42−) is prevalent. In weakly acid conditions, the dihydrogen phosphate ion (H2PO4−) is most common. In strongly acidic conditions, trihydrogen phosphate (H3PO4) is the main form (CHIBAN et al., 2012). In aqueous solution at 25 °C are:

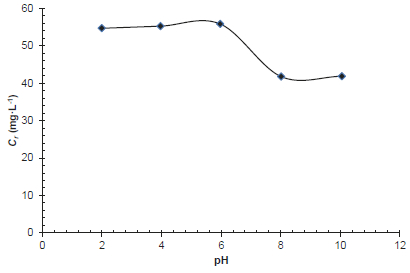
The pH of the aqueous solution is an important parameter which controls the adsorption process (KRATOCHVIL and VOLESKY, 1998). Thus, the effect of hydrogen ion concentration was examined for solutions at pH ranging from 2 to 10 at 25 °C and at fixed initial concentration ions Ci = 100 mg∙L-1. Figure 10 summarizes the uptake of phosphate anions at various pH by ISBM adsorbent.
Figure 10
Effect of pH on the removal of phosphate anions by the raw shrimp shells (Ci = 100 mg∙L-1, R = 25 g∙L-1, tc = 24 h, T = 25 °C)
Variation de la concentration d’ions phosphates retenus par le broyat de carapaces de crevettes à l’état brut en fonction du pH (Ci = 100 mg∙L-1, R = 25 g∙L-1, tc = 24 h, T = 25 °C)
It is evident that the removal of phosphate is constant at a pH range 2-6, and then decreases slightly while pH increases. The pH effect may be explained in relation to the competition effect between the hydroxide ions and mineral ions. At high pH values, the concentration of OH- far exceeds that of the mineral ions; hence these are bound to the adsorbent, leaving the mineral ions unbound as was explained in the paragraph 3.2.2 (CHIBAN, 2007; SOUDANI et al., 2009; ZHANG et al., 2010).
The results of the zero point of charge of the shrimp shells is found to be pHPZC = 8.1. This shows that at pH less than 8.1 the surface of the shrimp shells is predominated by positive charges while at pH greater than 8.1 the surface is predominated by negative charges. Thus, at pH < pHPZC, the surface has a high positive charge density; uptake of negatively charged phosphate ions would be high. At pH > pHPZC, the surface has a high negative charge density; uptake of negatively charged phosphate ions would be low.
The reason for good removal of orthophosphates at the lower pH is that the negative charge on the surface is reduced due to the excess of protons in solutions. As a result, the pH of the system decreases and the number of positively charged sites increase. A positively charged surface site on the raw shell shrimp favors the adsorption of the phosphate anions due to electrostatic attraction.
3.2.6 Effect of temperature
The effect of temperature (T) on the phosphate anions adsorption by the raw shrimp shells were studied at 20, 25, 30, 35, and 40 °C at 100 mg∙L-1 initial anions concentration and at natural pH, The results obtained, were plotted in Figure 11 for HPO42-, H2PO4- anions.
Figure 11
Effect of temperature on the removal of phosphate anions by the raw shrimp shells (R = 25 g∙L-1; tc = 24 h; Ci = 100 mg∙L-1)
Variation de la concentration d’ions phosphates retenus par le broyat de carapaces de crevettes à l’état brut en fonction de la température (R = 25 g∙L-1; tc = 24 h; Ci = 100 mg∙L-1)
These figures show that the concentration of phosphate anions adsorbed at different temperatures indicated the removal of these anions decreases with increasing of the temperature to stabilize from T = 30 °C. These results indicated also that the adsorption process of anions studied was exothermic. The adsorption capacity decreased with increasing of temperature. At T > 30 °C, the influence of temperature on the adsorption becomes negligible.
3.3 Comparison with published data
The application of low-cost and easily available materials in wastewater treatment has been widely investigated during recent years. Particularly, the phosphate adsorption on different materials has been widely studied during recent years. In order to situate our natural adsorbent among those used to remove orthophosphate from aqueous solutions, the orthophosphate anions adsorption capacity of Shrimp shells adsorbent was compared to the adsorption capacities of some other adsorbents reported in literature (Table 3). For example, NAMASIVAYAM and SANGEETHA (2004) examined the adsorption of phosphate onto coir pith carbon and they have found a 5.1 mg∙g-1 phosphate uptake capacity. OGUZ (2004) studied for the blast furnace slag the adsorption of phosphate ions and the adsorption capacity of phosphate was only 6.37 mg∙g-1 at an equilibrium time (te) of 60 min and a pH of 8.5. HUANG et al. (2008) studied the phosphate removal from wastewater using red mud. They found that all activated red mud samples show higher surface area and total pore volume as well as higher adsorption capacity for phosphate removal. The red mud with HCl treatment shows the highest adsorption capacity among all the red mud samples, giving adsorption capacity of 0.58 mg∙g-1 at pH 5.5 and 40 °C. Peat was also used by XIONG and MAHMOOD (2010) as an adsorbent without any pretreatment for studying the adsorption of phosphate from aqueous solution. They found that the maximum phosphate adsorption on peat was 8.91 mg∙g-1. It is obvious from these results that the adsorption affinity of Shrimp shells adsorbent towards phosphate ions is comparable or higher than other available adsorbents. The differences in phosphate anions maximum adsorption capacities are due to the properties of each adsorbent such as structure, surface area, polyphenolic groups, functional groups etc. It is proved that Shrimp shells could be considered as a promising material and one alternative source for low cost absorbents to remove phosphate ions from wastewaters.
Table 3
Comparison of the phosphate adsorption capacities onto shrimp shells with other natural adsorbents
Comparaison des quantités maximales d'ions orthophosphates adsorbées par différents matériaux
4. Conclusion
The results of this study indicate that ISBM is an effective adsorbent for removal of phosphate from aqueous solutions. At saturation, the adsorption capacities of phosphate ions by 1 gram of ISBM was found to be about 200 mg∙g-1 and 400 mg∙g-1 for HPO42- and H2PO4- respectively. It was found that the concentration of phosphate adsorbed on ISBM comparatively depends on pH, temperature and contact time of the solution. The removal of these anions decreases with increasing of the temperature to stabilize from T = 30 °C and the adsorption process was exothermic. Results indicated also that the removal of phosphate is constant at a pH range 2-6, and then decreases slightly while pH increases. Langmuir and Freundlich isotherms show the existence of several independent adsorption sites obeying to Langmuir. The Temkin isotherm suggests repulsive and weak electrostatic interactions.
Parties annexes
References
- AGYEI N.M., C.A. STRYDOM and J.H. POTGIETER (2002). The removal of phosphate ions from aqueous solution by fly ash, slag, ordinary Portland cement and related blends. Cement Concrete Res., 32 (12), 1889-1897.
- ALTUNDOGAN H.S. and F. TUMEN (2002). Removal of phosphorus from aqueous solutions by using bauxite. I: Effect of pH on the adsorption of various phosphates. J. Chem. Technol. Biot., 77, 77-85.
- BAILEY S.E., T.J. OLIN, R.M. BRICKA and D.D. ADRIAN (1999). A review of potentially low-cost sorbents for heavy metals. Water Res. 33 (11), 2469-2479.
- BANERJEE S. and M.C. CHATTOPADHYAYA (2013). Adsorption characteristics for the removal of a toxic dye, tartrazine from aqueous solutions by a low cost agricultural by-product. Arab. J. Chem., http://dx.doi.org/10.1016/j.arabjc.2013.06.005
- BENYOUCEF S. and M. AMRANI (2011). Adsorption of phosphate ions onto low cost Aleppo pine adsorbent. Desalination, 275 (1-3), 231-236.
- BHATNAGAR A. and M. SILLANPÄÄ (2009). Applications of chitin- and chitosan-derivatives for the detoxification of water and wastewater - A short review. Adv. Colloid Interfac., 152 (1-2), 26-38.
- BISWAS B.K., K. INOUE, K.N. GHIMIRE, S. OHTA, H. HARADA, K. OHTO and H. KAWAKITA (2007). The adsorption of phosphate from an aquatic environment using metal-loaded orange waste. J. Colloid Interface Sci., 312 (2), 214-223.
- CHIBAN M. (2007). Étude de l'adsorption en système statique (réacteur « batch ») et dynamique (membranes) d'ions métalliques, minéraux et autres polluants sur des biomatériaux inertes solides (végétaux secs) à partir de solutions modèles et d'eaux usées de la région d'Agadir. Ph.D. Thesis, Université Sidi Mohamed Ben Abdellah, Fez, Morocco, 238 p.
- CHIBAN M., A. SOUDANI, M. ZERBET and F. SINAN (2013). Wastewater treatment processes. In: Handbook of Wastewater Treatment: Biological Methods, Technology and Environmental Impact. Nova Science Publishers Inc., New York, USA, Chap. 10, pp. 249-262.
- CHIBAN M., M. ZERBET and F. SINAN (2012). Low-cost materials for phosphate removal from aqueous solutions. In: Phosphates: Sources, Properties and Applications. Nova Science Publishers Inc., New York, USA, Chap. 1, pp. 1-43.
- CHUBAR N.I, V.A. KANIBOLOTSKYY, V.V. TRELKO, G. GALLIOS, V.F. SAMANIDOU, T.O. SHAPOSHNIKOVA, I.Z. ZHURAVLEV and V.G. MILGRANDT (2005). Adsorption of phosphate ions on novel inorganic ion exchangers. Colloid Surface A, 255 (1-3), 55-63.
- DE LIMA A.C.A., R.F. NASCIMENTO, F.F. DE SOUSA, J.M. FILHO and A.C. OLIVEIRA (2012). Modified coconut shell fibers: A green and economical sorbent for the removal anions from aqueous solutions. Chem. Eng. J., 185-186, 274-284.
- DEL BUBBA M., C.A. ARIAS and H. BRIX (2003). Phosphorus adsorption maximum of sands for use as media in subsurface flow constructed reed beds as measured by the Langmuir isotherm, Water Res. 37, 3390-3400.
- DIVYA JYOTHI M., K. ROHINI KIRAN and K. RAVINDHRANATH (2012). Phosphate pollution control in waste waters using new bio-sorbents, Int. J. Water Res. Environ. Eng., 4 (4), 73-85.
- DOGAN M., M. ALKAN, A. TURKYILMAZ and Y. OZDEMIR (2004). Kinetics and mechanism of removal of methylene blue by adsorption onto perlite. J. Hazard. Mater., 109 (1-3), 141-148.
- EBERHARDT T.L., S.H. MIN and J.S. HAN (2006). Phosphate removal by refined aspen wood fiber treated with carboxymethyl cellulose and ferrous chloride. Bioresour. Technol., 97 (18), 2371-2376.
- FREUNDLICH H. (1906). Over the adsorption in solution. J. Phys. Chem., 57, 385-470.
- HO Y.S. and G. McKAY (1998). Sorption of dye from aqueous solution by peat. Chem. Eng. J., 70 (2), 115-124.
- HUANG W., S. WANG, Z. ZHU, L. LI, X. YAO, V. RUDOLPH and F. HAGHSERESHT (2008). Phosphate removal from wastewater using red mud. J. Hazard. Mater., 158, 35-42.
- ISMAIL Z. (2012). Kinetic study for phosphate removal from water by recycled date-palm wastes as agricultural by-products. Int. J. Environ. Stud., 69 (1), 135-149.
- JOHNSON E.L. and Q.P. PENISTON (1982). Utilization of shellfish waste for chitin and chitosan production. In: Chemistry and Biochemistry of Marine Food Products. (MARTIN R.E., C.G. FLICK, C.E. HEBARD AND D.K. WARD (Editors), Avi Publishing, Westport, CT, USA, Chap. 19, pp. 415-428.
- KARACA S., A. GÜRSES, M. EJDER and M. ACIKYLDIZ (2006). Adsorptive removal of phosphate from aqueous solutions using raw and calcinated dolomite, J. Hazard. Mater., 128 (2-3), 273-279.
- KARAGEORGIOU K., M. PASCHALIS and G.N. NASTASSAKIS (2007). Removal of phosphate species from solution by adsorption onto calcite used as natural adsorbent. J. Hazard. Mater., 139 (3), 447-452.
- KRATOCHVIL D. and B. VOLESKY (1998). Advances in the biosorption of heavy metals, Trends Biotechnol., 16 (7), 291-300.
- KRISHNAN K.A. and A. HARIDAS (2008). Removal of phosphate from aqueous solutions and sewage using natural and surface modified coir pith. J. Hazard. Mater., 152 (2), 527-535.
- KURITA K. (2006). Chitin and chitosan: Functional biopolymers from marine crustaceans. Mar. Biotechnol., 8 (3), 203-226.
- LAGERGREN S. and B.K SVENSKA (1898). Zur theorie der sogenannten adsorption geloester stoffe, Vaternskapsakad. Handlingar, 24 (4), 1-39.
- LANGMUIR I. (1916). The constitution and fundamental properties of solids and liquids. J. Am. Chem. Soc., 38 (11), 2221-2295.
- LI Y., C. LIU, Z. LUAN, X. PENG, C. ZHU, Z. CHEN, Z. ZHANG, J. FAN and Z. JIA (2006). Phosphate removal from aqueous solution using raw and activated red mud and fly ash. J. Hazard. Mater., 137, 374-383.
- LIU C., Y.Z. LI, Z.K. LUAN, Z.Y. CHEN, Z.G. ZHANG and Z.P. JIA (2007). Adsorption removal of phosphate from aqueous solution by active red mud. J. Environ. Sci., 19, 1166-1170.
- MEZENNER N.Y. and A. BENSMAILI (2009). Kinetics and thermodynamic study of phosphate adsorption on iron hydroxide-eggshell waste. Chem. Eng. J., 147 (2-3), 87-96.
- MOLLE P., A. LIENARD, A. GRASMICK, A. IWEMA and A. KABBABI (2005). Apatite as an interesting seed to remove phosphorus from wastewater in constructed wetlands. Water Sci. Technol., 51 (9), 193-203.
- NAMASIVAYAM C. and D. SANGEETHA (2004). Equilibrium and kinetic studies of adsorption of phosphate onto ZnCl2 activated coir pith carbon. J. Colloid Interface Sci., 280, 359-365.
- NGUYEN T.A.H., H.H. NGO, W. GUO and T.V. NGUYEN (2012). Phosphorous removal from aqueous solutions by agricultural by-products: A critical review. J. Water Sustain., 3, 193-207.
- OBSERVATOIRE NATIONAL DE L'ENVIRONNEMENT ET DU DEVELOPPEMENT DURABLE DU MAROC (2015). 3ème rapport sur l'état de l'environnement du Maroc : 2015. Ministère délégué auprès du ministre de l'énergie, des mines, de l'eau et de l'environnement, chargé de l'environnement, Maroc,187 p.
- OGUZ E. (2004). Removal of phosphate from aqueous solution with blast furnace slag. J. Hazard. Mater., 114, 131-137.
- OZACAR M. and I.A. SENGIL (2003). Adsorption of reactive dyes on calcined alunite from aqueous solutions. J. Hazard. Mater., 98 (1-3), 211-224.
- PROCHASKA C.A. and A.I. ZOUBOULIS (2006). Removal of phosphate by pilot vertical-flow constructed wetlands using a mixture of sand and dolomite as substrate. Ecol. Eng., 26, 293-303.
- RIAHI K., S. CHAABANE and B. BEN THAYER (2013). A kinetic modeling study of phosphate adsorption onto Phoenix dactylifera L. date palm fibers in batch mode. J. Saudi Chem. Soc., http://dx.doi.org/10.1016/j.jscs.2013.11.007
- ROBERTS G.A.F. (1992). Chitin Chemistry. Palgrave Macmillan, London, UK, 368 p.
- RODIER J., B. LEGUBE, N. MERLET and R. BRUNET (2009). L'analyse de l'eau : eaux naturelles, eaux résiduaires, eaux de mer. 9ème édition, Collection : Technique et ingénierie, Dunod, Paris, France, 1600 p.
- SAAD R., K. BELKACEMI and S. HAMOUDI (2007). Adsorption of phosphate and nitrate anions on ammonium-functionalized MCM-48: effects of experimental conditions. J. Colloid. Interface Sci., 311(2), 375-381.
- SAKADEVAN K. and H.J. BAVOR (1998). Phosphate adsorption characteristics of soils, slags and zeolite to be used as substrates in constructed wetland systems. Water Res., 32, 393-399.
- SOUDANI A., M. CHIBAN, H. EDDAOUDI, F. SINAN, S. TAHROUCH and M. PERSIN (2009). Mineral ions adsorption onto inert solid biomaterials of vegetal origin: Study extended to the strong limiting concentrations leading to the total saturation of adsorbent. In: Current Focus on Colloids and Surfaces, LI S. (Editor), Transworld Research Network, 37/661, pp. 209-225.
- TEMKIN M.J. and V. PYZHEV (1940). Application of Temkin adsorption isotherm. Acta Physiochim., 12, 217-222.
- VAN HOLDE K.E., W.C. JOHNSON and P.S. HO (1998). Principles of Physical Biochemistry. Prentice Hall, Upper Saddle River, NJ, USA, 710 p.
- VIESSMAN W. Jr. and M.J. HAMMER (2005). Water Supply and Pollution Control. Pearson Prentice Hall, Upper Saddle River, NJ, USA, 876 p.
- WAHAB M.A., R. BEN HASSINE and S. JELLALI (2011). Posidonia oceanica (L.) fibers as a potential low-cost adsorbent for the removal and recovery of orthophosphate. J. Hazard. Mater., 191, 333-341.
- WAN NGAH W.S. and M.A. HANAFIAH (2008). Removal of heavy metal ions from wastewater by chemically modified plant wastes as adsorbents: A review. Bioresour. Technol., 99 (10), 3935-3948.
- XIONG J.B. and Q. MAHMOOD (2010). Adsorptive removal of phosphate from aqueous media by peat. Desalination, 259, 59-64.
- YE H., F., CHEN and Y. SHENG (2006). Adsorption of phosphate from aqueous solution onto modified palygorskites. Sep. Purif. Technol., 50, 283-290.
- YOUCEF L. and S. ACHOUR (2005). Élimination des phosphates par des procédés physico-chimiques. Larhyss J., 4, 129-140.
- ZENG L., X. LI and J. LIU (2004). Adsorptive removal of phosphate from aqueous solutions using iron oxide tailings. Water Res., 38, 1318-1327.
- ZHANG J., Z. SHEN, W. SHAN, Z. CHEN, Z. MEI, Y. LEI and W. WANG (2010). Adsorption behavior of phosphate on lanthanum(III) doped mesoporous silicates material. J. Environ. Sci., 22 (4) 507-511.
Liste des figures
Figure 1
Schematization of the raw shrimp shells (JOHNSON and PENISTON, 1982; KURITA, 2006)
Schématisation de carapaces de crevettes à l’état brut (JOHNSON et PENISTON, 1982; KURITA, 2006)
Figure 2
Scanning electron microscope image of the raw shrimp shells (particle size < 500 μm)
Image par microscopie électronique à balayage (MEB) de la poudre du broyat de carapaces de crevettes à l’état brut (granulométrie < 500 µm)
Figure 3
Energy dispersive X-ray spectrum of raw shrimp shell particles (particle size < 500 μm)
Spectre de rayons X à dispersion d'énergie de carapaces de crevettes à l’état brut (granulométrie < 500 µm)
Figure 4
Infrared spectrum of the raw shrimp shells in KBr
Spectre infrarouge de carapaces de crevettes à l’état brut dans KBr
Figure 5
Variation of the phosphate anion concentrations adsorbed by the raw shrimp shells as a function of the m/V ratio (R): Ci = 100 mg∙L-1, T = 25 °C and tc = 24 h
Variation de la concentration d'ions phosphates retenus par le broyat de carapaces de crevettes à l’état brut en fonction du rapport R (Ci = 100 mg∙L-1; T = 25 °C; tc = 24 h)
Figure 6
Effect of contact time on the removal of the phosphate anions by the raw shrimp shells (R = 25 g∙L-1; pH = 7.55; T = 25 °C)
Variation de la concentration d’ions phosphates retenus par le broyat de carapaces de crevettes à l’état brut en fonction du temps de contact (R = 25 g∙L-1; pH = 7,55; T = 25 °C)
Figure 7
Effect of initial concentration on the removal of HPO42- and H2PO4- anions from single ion solutions by raw shrimp shells (R = 25 g∙L-1; T = 25 °C; tc = 6 h > te = 2 h)
Variation de la concentration d’ions phosphates retenus par le broyat de carapaces de crevettes à l’état brut en fonction de la concentration initiale (R = 25 g∙L-1; T = 25 °C; tc = 6 h > te = 2 h)
Figure 8
Plot of inverse values of Cr vs. Ci corresponding to the data shown in Figure 7
Variation de 1/Cr en fonction de 1/Ci
Figure 9
Isotherm plots for the adsorption of HPO42- anions by the raw shrimp shells
Isothermes d'adsorption de HPO42- sur le broyat de carapaces de crevettes à l’état brut
Figure 10
Effect of pH on the removal of phosphate anions by the raw shrimp shells (Ci = 100 mg∙L-1, R = 25 g∙L-1, tc = 24 h, T = 25 °C)
Variation de la concentration d’ions phosphates retenus par le broyat de carapaces de crevettes à l’état brut en fonction du pH (Ci = 100 mg∙L-1, R = 25 g∙L-1, tc = 24 h, T = 25 °C)
Figure 11
Effect of temperature on the removal of phosphate anions by the raw shrimp shells (R = 25 g∙L-1; tc = 24 h; Ci = 100 mg∙L-1)
Variation de la concentration d’ions phosphates retenus par le broyat de carapaces de crevettes à l’état brut en fonction de la température (R = 25 g∙L-1; tc = 24 h; Ci = 100 mg∙L-1)
Liste des tableaux
Table 1
Parameters of pseudo-first order and pseudo-second order kinetic models for HPO42- and H2PO4- ions
Paramètres des modèles cinétiques du pseudo-premier ordre et pseudo-second ordre pour les ions HPO42- et H2PO4-
Table 2
Isotherm model parameters and correlation coefficients for HPO42- and H2PO4- ions
Paramètres et valeurs des coefficients de corrélation des isothermes pour les ions HPO42- et H2PO4-
Table 3
Comparison of the phosphate adsorption capacities onto shrimp shells with other natural adsorbents
Comparaison des quantités maximales d'ions orthophosphates adsorbées par différents matériaux