Résumés
Abstract
Wastewater conductivity has been monitored for extended periods of time by in situ probes and on grabbed samples in four communities (from 1,000 to 350,000 PE). In parallel, the concentrations of the main ionic contributors, such as calcium, sodium, potassium, magnesium, ammonium, ortho-phosphate, chloride and sulphate have been measured and their variations with respect to time compared to human activity patterns. It appears that sodium, potassium, ammonium and ortho-phosphate, which contribute to about 34% to wastewater conductivity, exhibit diurnal variations in phase with human activity evaluated by absorbance at 254 nm. However calcium (≈ 22% of wastewater conductivity) is out‑of-phase. This release, ahead of the one of other cations and anions, could be related to sewer concrete corrosion or to groundwater infiltration. The combination of these different ionic contributions creates a conductivity pattern which cannot be easily related to human activity. It makes difficult to integrate conductivity in a monitoring system able to detect ion-related abnormalities in wastewater quality.
Keywords:
- Conductivity,
- corrosion,
- urine,
- calcium,
- potassium,
- infiltration
Résumé
Les variations de la conductivité d’eaux usées urbaines ont été suivies sur de longues durées a l’aide de sondes placées in situ en entrée d’installations de traitement et sur des échantillons prélevés automatiquement. Quatre communautés (entre 1 000 et 350 000 habitants) ont été choisies pour cette étude. On a également dosé sur les échantillons les principaux ions (calcium, sodium, potassium, magnésium, ammonium, ortho-phosphates, chlorures et sulfates). Il apparait que le sodium, le potassium, l’ammonium et les ortho-phosphates contribuent pour 34 % a la conductivité des eaux usées et présentent des variations journalicres en phase avec la pollution carbonée soluble, estimée a partir de l’absorbance a 254 nm. Par contre, le calcium, qui contribue pour 22 % a la conductivité, présente un déphasage qui peut ztre du a son transport dans le réseau d’assainissement du fait de la corrosion des conduites en béton ou a des infiltrations d’eaux claires. Finalement, la combinaison de ces différentes contributions ioniques conduit a une variabilité de la conductivité qu’il n’est pas facile de lier a l’activité humaine, et donc a des rejets accidentels dans le cadre d’un systcme de détection de variation anormale de la qualité des eaux usées.
Mots clés:
- Conductivité,
- corrosion,
- urine,
- calcium,
- potassium,
- infiltration
Corps de l’article
1. Introduction
Domestic wastewater treatment systems of any kind (continuous or batch activated sludge reactors, anaerobic digesters, lagoons, etc.) are subjected to variations in flow and load due mainly to predictable changes in urban activity (normal diurnal cycle, week-ends and holidays). But accidental releases of industrial pollution and weather-related events (storm, long rain periods) may also occur. They can be considered as critical situations, which require steps to be taken to protect especially the biological stage: their rapid detection can be used in the control strategy of the system to trigger the opening of gates and to direct part or totality of the flow towards a temporary storage tank. As regulations require the treatment of all incoming wastewater in suitable facilities, the protection of the biological stages by detection of critical events is more and more necessary.
Wastewater characteristics are generally difficult to obtain rapidly. Automated chemical and biochemical analysis is proposed for COD, ammonium, nitrate, etc. and devices based on spectrophotometry are also available (HOCHEDLINGER et al., 2005; LANGERGRABER et al., 2003). For toxics it appears unrealistic to organize a specialized detection at the plant inlet as well as in the sewer network. The spectrum of possible components is very large: heavy metals, fuels, solvents, pesticides, herbicides, detergents, road de-icing products, etc. Respirometry-based systems are available for wastewater treatment plants (LE BONTE et al., 2005) but they are difficult to use along the sewer system, especially as many require the use of activated sludge. Conductivity and pH probes belong to a class of sensors that can be easily placed in situ either at the inlet or outlet of a wastewater treatment plant (KIM OANH and BENGTSSON, 1995) or further upstream in the sewer system to provide information on wastewater ionic content. Such a project has been initiated by the Greater Nancy (France) to implement early-warning systems based mainly on electrochemical probes in various locations of the sewer network (GRAPIN, 2004). But conductivity variations have been found difficult to understand. More recently, similar projects have been started in Austria and the Netherlands (SCHILPEROORT et al., 2006). The variations of the conductivity/calcium ratio have been proposed to detect easily pollution in rivers by untreated sewage (NIREL and REVACLIER, 2003). The conductivity c of a liquid sample is a linear function of the concentrations (Ci) of the n ions present:
where λi is the mobility of ion i and zi its electrical charge. Its variation will be related to cations and anions (mostly inorganic) present in the wastewater. In the case of release of high concentrations of ions (bases, acids, salts) the conductivity variations could be used to warn the plant operators and help to locate the discharge point in the sewer network. In order to evaluate whether conductivity could be used to detect changes in (waste)water composition, reference patterns should be obtained under dry weather conditions. For that purpose, the relation between the wastewater concentration of main ions and conductivity has been examined over extended periods of time in communities of different sizes.
2. Materials and Methods
Wastewater grabbed samples have been collected at four different locations in the north-eastern part of France with auto-samplers (Sigma 900P, Hach Company, Loveland, Colorado for Nancy and Pont-a-Mousson, ASP-Station 2000, Endress+Hauser for Colombey-les-Belles and Villey-Saint-Etienne).
In Nancy-Maxéville (WWTP capacity = 350,000 PE), the device was installed on a loop providing primary settled wastewater, as shown in figure 1. On the same loop was installed a well-mixed tank, continuously fed with the same wastewater, in which were located a conductivity probe (CDC 565, Radiometer Analytical, Villeurbanne, France) and a pH (Type 06 242 040, Prolabo, Paris, France) probe. These probes were connected respectively to a PHM220 pHmeter and a CDM210 conductimeter (Radiometer Analytical, Villeurbanne, France). The pHmeter and the conductimeter were themselves connected via a RS232 multiplexer (Black Box Network Products n.v., Zaventem, Belgium) to a PC. A procedure developed under HPView (Hewlett-Packard) enabled the reading and storage of pH and conductivity values every 5 min. Data were collected over two periods, in winter and in summer. Calibration was checked weekly.
Figure 1
Experimental set-up at the Nancy-Maxéville wastewater treatment plant.
Site expérimental de la station d’épuration de Nancy-Maxévill.
In Pont-a-Mousson (WWTP capacity = 16,200 PE), Colombey-les-Belles (1,200 PE) and Villey-Saint-Etienne (1,050 PE), raw wastewater samples were collected at the inlet of the wastewater works. The pH and conductivity of these grabbed samples were measured as above. Data were collected in spring for Pont-a-Mousson and in summer for Colombey-les-Belles and Villey-Saint-Etienne.
After filtration of all grabbed samples (on paper filter of pore diameter ≈ 7 µm), calcium, sodium and magnesium were determined by atomic absorption and potassium by atomic emission with a Varian AA20 spectrophotometer, according to the manufacturer’s guidelines. Adequate dilution with de-ionised water was performed with a Hamilton auto-diluter in order to be in the linear response range of the device for each element. For the analysis of anions (sulphate, chloride, nitrate, ortho-phosphate), the samples were filtrated again (pore diameter = 0.45 µm) and analyzed with a Dionex HPLC, according to the manufacturer’s guidelines. The overall precision is estimated to less than 5% for these cations and anions. Ammonium was determined by the Nessler method modified to accommodate small samples (Hach method 380 on DR/2000). Ortho-phosphate was also determined spectrophotochemically (Hach method 490 on DR/2000). The overall precision is estimated to less than 5% for ammonium and ortho-phosphates. Absorbance at 254 nm (A254) was measured on filtrated samples with an Anthelie Light spectrophotometer (Secomam, Domont, France). It is used as a surrogate parameter for COD (THOMAS et al., 1993), encompassing, due to the paper filter pore size, dissolved COD (< 0.45 µm) and colloidal COD (MRKVA, 1983). A coefficient of correlation larger than 0.84 was obtained between A254 and COD (Hach method 435) (Figure 2) confirming the results found by WU et al. (2006).
Figure 2
Correlation between absorbance at 254 nm (A254) and COD.
Corrélation entre l’absorbance a 254 nm (A254) et la DCO.
3. Results and Discussion
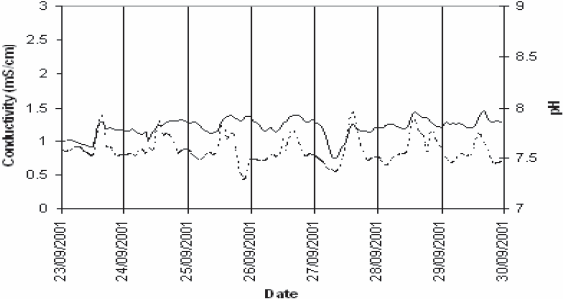
Figure 3 presents three examples of one-week monitoring of the variations of conductivity and pH in Nancy, Colombey-les-Belles and Villey-Saint-Etienne in summer. For Colombey and Villey the weather was dry but some rainfall had occurred two days before sampling started in Villey, which may have caused some disturbances on the first samples. Some small rainfalls occurred in Nancy. Nancy is a large community, with many small (but no large) industries of various natures, such as laundries, house-painting businesses, hardware stores, car-repairs, restaurants, hospitals, and a complex sewer system. Diurnal patterns of organic and nitrogen pollutions are generally reported at the inlet of municipal wastewater treatment plants, reflecting the diurnal activity of inhabitants (HENZE et al., 2002; METCALF and EDDY, 1991; PETERSEN et al., 2002). In Nancy a diurnal pattern of variation of conductivity and pH is clearly visible. It is not so for the villages: it should be mentioned that Villey does not have any industry and Colombey has only small ironmongery and a small industrial bakery. To explain such variations, it is necessary to look more closely to the variations of cations and anions. It can be seen also that the variations are largely dampened for Nancy due to the size of the sewer network (750 km), much larger that the ones of the villages (between 10 and 20 km).
Figure 3
Examples of variations of conductivity (—) and pH (---) for three different communities in summer: (a) Nancy; (b) Colombey-les-Belles and (c) Villey-Saint-Etienne.
Exemples de variations de la conductivité (—) et du pH (---) pour trois sites expérimentaux en été; (a) Nancy; (b) Colombey-les-Belles and (c) Villey-Saint-Etienne.
(a)
(b)
(c)
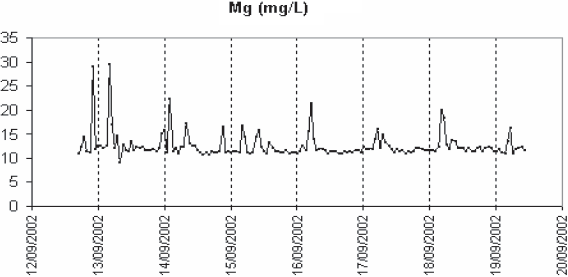
It can be seen in figure 4, which presents data collected in Nancy in winter, that the variations can be very different from one ion to another. Calcium and potassium exhibit diurnal variations. Potassium concentration is maximal when the soluble organic pollution (monitored by the surrogate parameter A254) is maximal. It is the contrary for calcium, which increases at the end of the night. In this series magnesium kept increasing. A diurnal variation can be observed for sodium, but it is masked here by the large peak at 220 mg/L, which is due to rainfall and washout of road de-icing salt (which is generously spread on the streets in the North-East of France) in the sewer network. A peak of chloride can be observed at the same time. The concentration of nitrates is low, although slightly higher than the values reported in literature (0.5 mg N‑NO3/L in HENZE et al., 2002). This could be due to some nitrification occurring in the sewer system that could be favoured by the steep slopes in many parts of Greater Nancy. Diurnal variations can also be observed.
Figure 4
Variations of the concentrations (open symbols) of some anions (chlorides and nitrates) and cations (sodium, potassium, magnesium and calcium) in Nancy (winter season). (- - - ) A254: (a) Ca; (b) Chlorides; (c) K; (d) Mg; (e) Na; (f) Nitrates.
Variations des concentrations (symboles ouverts) de quelques anions (chlorures ry nitrates) et cations (sodium, potassium, magnésium et calcium) a Nancy (en hiver). (- - - ) A254. (a) Ca; (b) Chlorides; (c) K; (d) Mg; (e) Na; (f) Nitrates.
(a)
(b)
(c)
(d)
(e)
(f)
Table 1 summarizes the drinking water composition which can be used as a reference to analyze the wastewater data. In table 2, data on urine composition have been collected from literature (UDERT et al., 2003a, 2003b). Urine is the main source of ammonia in domestic wastewater as urea is easily hydrolyzed according to the following reaction (UDERT et al., 2003b):
The ratio of each species with respect to total potential ammonia (NH+4hyd) (η) has been calculated for urine and average Nancy wastewater. Urine contains also large amounts of potassium, sodium and chloride. Due to high time-correlation between ammonia and potassium, it is likely that a large part of the potassium released in the sewer is related to urine. Furthermore ηK,urine is of the same order of magnitude than ηK,wastewater. The average sodium content in wastewater is higher than in the drinking water but ηNa,urine is lower than ηNa,wastewater. As stated in ERIKSSON et al. (2002), sodium is often included in powder laundry detergents as a counterion for sulphate (diluting agent), phosphate, silicate, carbonate or borates (builders). Grey-water from washing activities is therefore a source of sodium. During winter season, de-icing salt is also the source of sudden peaks of sodium. In Nancy, the calcium content in wastewater is much higher than in drinking water. As urine contains almost no calcium, its origin should be found elsewhere.
Table 1
Drinking water characteristics (average concentrations).
Caractéristiques des eaux potables (concentrations moyennes).
Location |
Na (mg/L) |
K (mg/L) |
Mg (mg/L) |
Ca (mg/L) |
Cl- (mg/L) |
SO42- (mg/L) |
|---|---|---|---|---|---|---|
Nancy |
12 |
2.5 |
11 |
21 |
20 |
97 |
Pont-a-Mousson |
5.6 |
4.9 |
15 |
83 |
- |
- |
Villey-Saint-Etienne |
5.7 |
3.6 |
12 |
108 |
- |
- |
Colombey-les-Belles |
5 |
1.5 |
4 |
130 |
- |
- |
Table 2
Comparison of urine and wastewater composition.
Comparaison des compositions de l’urine et des eaux usées
|
Litterature data (UDERT et al. 2003a, 2003b) |
ηurine |
ηwastewater |
||
|---|---|---|---|---|---|
|
Concentration (mg/L) |
CV% |
Range (mg/L) |
||
NH4+ (mgN/L) |
480 |
29 |
|
|
|
Urea (mgN/L) |
7700 |
20 |
|
|
|
NH4+hyd(mgN/L) |
8180 |
|
|
|
|
Phosphate (mgP/L) |
740 |
14 |
|
0.09 |
0.33 |
Calcium (mg/L) |
190 |
|
|
0.02 |
3.3 |
Sodium (mg/L) |
2800 |
|
1800‑5800 |
0.34 |
2.8 |
Potassium (mg/L) |
2200 |
|
1300‑3100 |
0.27 |
0.4 |
Magnesium (mg/L) |
100 |
21 |
|
0.01 |
0.3 |
Chloride (mg/L) |
3800 |
|
2300‑7700 |
0.46 |
3.8 |
Sulphate (mg/L) |
1500 |
29 |
|
0.18 |
3.3 |
Table 3 summarizes the range of variations of conductivity as well as calcium, potassium, magnesium and sodium concentrations for the four sampling sites. In table 4, the contributions of the different ions to the total conductivity have been roughly estimated based on the average concentrations measured in the four sites in winter and summer. More accurate evaluation will require extended sampling sessions and the present work should assess the real need for. In Nancy, which is the only location investigated in two different seasons, calcium varies between 70 and 100 mg/L in winter and between 50 and 70 mg/L in summer (out of rain events which decrease significantly the concentrations by dilution). The major contributors are calcium and chloride. Potassium contributes less than 2%. Figure 5 compares the measured conductivity and the conductivity estimated from the ion concentrations for Villey-Saint-Etienne. In that village, calcium and sodium are the main cations. The large peaks observed at the beginning of the campaign are difficult to explain with respect to the population normal activity (Figure 6). They might be related to the rain event which occurred previously. A clear periodicity can be observed for potassium. A first maximum is observed between 9:00 am and 11:00 am every morning. A second maximum is visible in the afternoon, when inhabitants are coming back from work. For calcium, sodium and magnesium, it is more difficult to figure out the relationship between their variations and the activity of the inhabitants. Obviously the early morning peaks for calcium and magnesium are occurring before the potassium peak. This explains the erratic variations of conductivity seen in figure 3. In Colombey (Figure 6), diurnal variations could be observed for potassium and magnesium, and to some extent for sodium (in spite of two high peaks on the Saturday morning which could be related to the intensive use of laundry machines). The variations of calcium are difficult to explain by anthropogenic reasons. But again, it appears that the early morning peaks for calcium and magnesium occur before the potassium peak.
Table 3
Range of variations of conductivity and concentrations in calcium, potassium, sodium and magnesium at the four sampling sites.
Domaine de variations de la conductivité et des concentrations en calcium, potassium, sodium et magnésium pour les quatre sites expérimentaux.
|
Conductivity (µS/cm) |
Calcium (mg/L) |
Sodium (mg/L) |
Magnesium (mg/L) |
Potassium (mg/L) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Min |
Max |
Min |
Max |
Min |
Max |
Min |
Max |
Min |
Max |
Nancy |
750 |
1450 |
65 |
101 |
51 |
217 |
7 |
9 |
7 |
13 |
Pont-a-Mousson |
190 |
1860 |
33 |
136 |
19 |
194 |
2 |
23 |
4.7 |
67 |
Villey-Saint-Etienne |
680 |
2840 |
56 |
306 |
41 |
457 |
9.2 |
30 |
12 |
59 |
Colombey-les-Belles |
900 |
2380 |
23 |
173 |
45 |
261 |
4 |
9 |
10 |
30 |
Table 4
Contributions of the various ions to total conductivity.
Contributions des différents ions à la conductivité totale.
Ion |
λ (mS.m2/mol) |
z |
Average concentration (mol/m3) |
Average conductivity by element (µS/cm) |
Contribution to total conductivity (%) |
|---|---|---|---|---|---|
Cl- |
7.63 |
1 |
3.78 |
28.8 |
25.9 |
Ca2+ |
5.95 |
2 |
2.07 |
24.6 |
22.1 |
Na+ |
5.01 |
1 |
3.09 |
15.5 |
13.9 |
SO42- |
8 |
2 |
8.54 10‑1 |
13.7 |
12.3 |
NH4+ |
7.34 |
1 |
1.8 |
13.2 |
11.9 |
PO43- |
9.28 |
3 |
2.68 10‑1 |
7.5 |
6.7 |
Mg2+ |
5.31 |
2 |
3.29 10‑1 |
3.5 |
3.1 |
K+ |
7.35 |
1 |
0.26 |
1.9 |
1.7 |
Total |
|
|
|
|
97.6 |
Figure 5
Comparison of measured (- - - ) and estimated (—) conductivities for Villey-Saint-Etienne.
Comparaison des conductivités mesurées (- - - ) et estimées (—) pour Villey-Saint-Etienne.
Figure 6
Calcium, potassium, magnesium and sodium: (a), (b), (c), (d) in Colombey-les-Belles; (e), (f), (g), (h) in Villey-Saint-Etienne.
Calcium, potassium, magnésium et sodium : (a), (b), (c), (d) in Colombey-les-Belles; (e), (f), (g), (h) in Villey-Saint-Etienne.
(a)
(b)
(c)
(d)
(e)
(f)
(g)
(h)
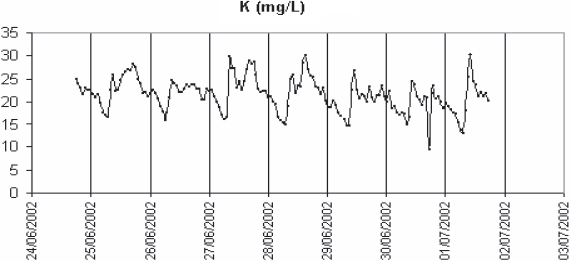
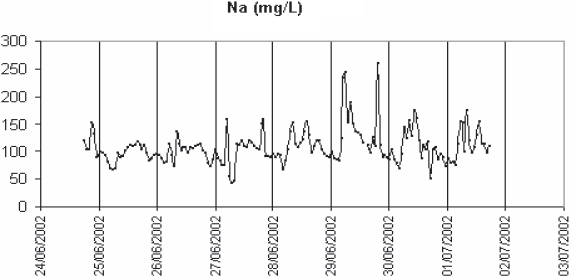
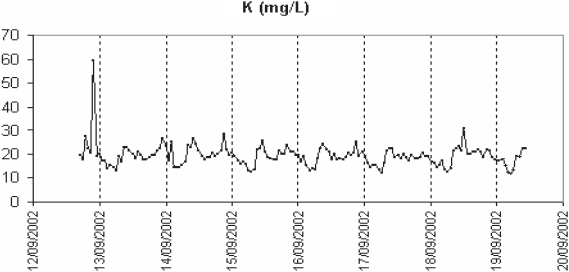
In order to confirm the out-of-phase variations of potassium and calcium, repeated long-term monitoring sessions of calcium and potassium were performed on the Nancy settled wastewater in summer and winter. One of them is shown in figure 7. Except during rain events when both concentrations drop sharply as well as conductivity, the calcium rises before potassium in the morning. It is not so much potassium which counter-balances calcium in conductivity than ammonia (which is highly correlated to potassium). As calculated in table 4, sodium, ammonia and ortho-phosphate represent about 32% of the total conductivity. One reason of the presence of calcium at the end of nights could be the corrosion of the mains, made of concrete, in which wastewater is flowing. Concrete contains mainly calcium aluminate, silicate and hydroxide. Depending upon the physico-chemical conditions, calcium can be released into the sewer system. pH is much lower at night, when ammonia, the major contributor to its balance, is minimum. The sewage flow is also minimal and anaerobic conditions favour the reduction by sulphate-reducing bacteria of S-containing substances into H2S. In the sewer gas phase, H2S is transformed into sulphuric acid which reacts with the calcium components of concrete (DE BELIE et al., 2004; ROBERTS et al., 2002). Part of calcium is incorporated in sewer sediment particles such as anapaite, whitlokite (containing also magnesium) and apatite. Apatite has been detected in Nancy sewer system (EL SEMRANI et al., 2004). The soluble fraction of calcium is transported to the wastewater treatment plant. The arrival of the calcium just ahead of the ammonia and potassium in the morning would result from the first-flush effect described by KREBS et al. (1999) but at a lower scale as it is governed by the rapid transition between night and day flows. Another hypothesis is related to the possible infiltration into the sewer system of groundwater rich in calcium. For comparison, groundwater quality data have been extracted from the Rhin-Meuse Basin Waterboard database (http://www.eau-rhin-meuse.fr) (Table 5) for piezometers the closest to the sampling sites. Tomblaine data are probably obtained at a too large depth to be really representative of the situation in the Greater Nancy sewer system. The high conductivity and sodium content at this location are related to the underground salt layer. Sparse data obtained closer to the ground layer give calcium values between 100 and 150 mg/L (personal communication from B. Lartiges, LEM) which could indeed explain the higher values observed in the wastewater samples in the night. The potential calcium flux, estimated from the wastewater flowrate at the inlet of the plant and the calcium data, doubles between night and day (150 (min.) to 300 (max.) kg/h), indicating anyway an anthropogenic source of calcium during the day. For Pont-a-Mousson, the maximal calcium value is close to the groundwater average value, which is in favour of the infiltration hypothesis. In the case of Colombey-les-Belles and Villey-Saint-Etienne, night calcium values are higher than the groundwater values. This leaves open the possible effect of pipe corrosion for these two sites. In conclusion it is difficult to select one hypothesis or the other based on the available data.
Figure 7
Long-range monitoring of settled wastewater in Nancy. Potassium (- - -), calcium (—) and conductivity (thick line).
Suivi sur une longue période de l’eau usée aprcs décantation primaire a Nancy. Potassium (- - -), calcium (—) et conductivité (trait épais).
Table 5
Groundwater characteristics near the four sampling sites. Data from the Rhin-Meuse Basin Water Board. Coefficient of Variation (CV) calculated on 35 data points for Francheville and Colombey-les-Belles (except for Mg: 33 data points), 6 data points for Tomblaine and 18 data points for Loisy over five years (2000 to 2005).
Caractéristiques des eaux souterraines prcs des quatre sites expérimentaux Données de l’Agence de l’Eau Rhin-Meuse. Coefficient de variation (CV) calculés sur 35 données pour Francheville et Colombey-les-Belles (sauf Mg: 33 données), 6 données pour Tomblaine et 18 données pour Loisy sur cinq ans (2000 to 2005).
|
Francheville (5 km from Villey-Saint-Etienne) (Dogger limestone) |
Colombey-les-Belles (Dogger limestone) |
Tomblaine (Greater Nancy) (Lower Trias sandstone) |
Loisy (5 km from Pont-a-Mousson) (Moselle alluvium) |
||||
|---|---|---|---|---|---|---|---|---|
Depth |
65 m |
0 m |
708 m |
8 m |
||||
|
Average |
CV (%) |
Average |
CV (%) |
Average |
CV (%) |
Average |
CV (%) |
Conductivity (µS/cm) |
638 |
15 |
468 |
20 |
1733 |
10 |
946 |
20 |
Potassium (mg/L) |
4.6 |
16 |
0.92 |
34 |
14.7 |
6.4 |
2 |
100 |
Magnesium (mg/L) |
26 |
12 |
4.8 |
100 |
21 |
10 |
10 |
17 |
Calcium (mg/L) |
68 |
8 |
102 |
16 |
84 |
10 |
135 |
12 |
Sodium (mg/L) |
56 |
9 |
6 |
50 |
246 |
18 |
44 |
37 |
4. Conclusions
Wastewater conductivity has been monitored for extended periods of time in four communities of different size range (from 1,000 to 350,000 PE) and socio-economical profile. In parallel, the concentrations of the main ionic contributors, such as calcium, sodium, potassium, magnesium, ammonium and ortho-phosphate have been measured and their variations with respect to time compared to human activity patterns. It appears that, if sodium, potassium, ammonium and ortho-phosphate, which contribute to about 34% to wastewater conductivity, exhibit diurnal variations in phase with human activity evaluated by absorbance at 254 nm, calcium (≈ 22% of wastewater conductivity) is out-of-phase. Its release might be related to sewer concrete corrosion or to groundwater infiltration but more experiments should be run to select the proper hypothesis, especially in locations with different groundwater characteristics. The combination of these different ionic patterns creates a conductivity pattern which does not facilitate its relation to a reference human activity that could be used in an automated system to detect abnormalities in wastewater quality. If events such as large rainfalls, which can cause in winter an increase of the conductivity by washout of de-icing salt and during the rest of the year a decrease by dilution, toxic spills linked to the release of ionic species might be difficult to detect.
Parties annexes
Acknowledgements
The authors wish to thank GEMCEA (Groupement pour l’Évaluation des Mesures en Continu dans les Eaux et en Assainissement), the Rhin-Meuse Water Basin Agency, the municipalities of Colombey-les-Belles, Villey-Saint-Etienne, Pont-a-Mousson and their waterworks staff, the Greater Nancy Council, for their help and sponsorship. Thanks also to C. Edet, A. Descousse, F. Centonze and S.Chanel for their participation to the sampling and analysis series and to E. Tisserand and P. Mocellin of LIEN-Henri Poincaré University (Vandoeuvre-les-Nancy, France) for developing the data logging software, to B. Lartiges (Laboratoire Environnement et Minéralurgie (LEM), INPL-CNRS) for helpful discussion.
Références bibliographiques
- DE BELIE N., J. MONTENY, A. BEELDENS, E. VINCKE, D. VAN GEMERT and W. WERSTRAETE (2004). Experimental research and prediction of the effect of chemical and biogenic sulphuric acid on different types of commercially produced concrete sewer pipes. Cem. Concr. Res., 34, 2223-2236.
- BÖHM M., J. DEVINNY, F. JAHANI and G. ROSEN (1998). On a moving-boundary system modelling corrosion in sewer pipes. Appl. Math. Comput., 92, 247-269.
- EL SAMRANI A.G., B.S. LARTIGES, J. GHANBAJA, J. YVON and A. KOHLER (2004). Trace element carriers in combined sewer during dry and wet weather: an electron microscope investigation. Water Res., 38, 2063-2076.
- ERIKSSON E., K. AUFFARTH, M. HENZE and A. LEDIN (2002). Characteristics of grey wastewater. Urban Water, 4, 85–104.
- GRAPIN G. (2004). Wastewater quality: from continuous measurement to pollution alert. PhD thesis. National Polytechnical Institute of Lorraine, Nancy, France.
- HENZE M., P. HARREMOES, J. COUR JANSEN and E. ARVIN (2002). Wastewater treatment: biological and chemical processes, 3rd Ed. Springer, Heidelberg, 430 p.
- HERNANDEZ M., E.A. MARCHAND, D. ROBERTS and J. PECCIA (2002). In situ assessment of active Thiobacillus species in corroding concrete sewers using fluorescent RNA probes. Int. Biodeterior. Biodegrad., 49, 271-276.
- HOCHEDLINGER M., G. GRÜBER and H. KAINZ (2005). Assessment of spill flow emissions on the basis of measured precipitation and waste water data. Atmos. Res., 77, 74-87.
- KIM OANH N.T. and G.E. BENGTSSON (1995). Development of a wastewater monitoring program incorporated into process control for mitigation of chemical and fiber loss from the Bai Bang Paper Company (BAPACO), a bleached kraft pulp and paper mill in Vietnam. Resour. Conserv. Recycl., 14, 53-66.
- KREBS P., P. HOLZER, J.L. HUISMAN and W. RAUCH (1999). First flush of dissolved compounds. Water Sci. Technol., 39, 55-62.
- LANGERGRABER G., N. FLEISCHMANN and F. HOFSTÄDTER (2003). A multivariate calibration procedure for UV/VIS spectrometric quantification of organic matter and nitrate in wastewater. Water Sci. Technol., 47, 63-71.
- LE BONTE S., O. POTIER and M.N. PONS (2005). Toxic event detection by respirometry and adaptive principal components analysis, Environmetrics, 16, 1-13.
- METCALF and EDDY (1991). Wastewater engineering: Treatment, disposal and reuse. 3rd Edition, Mc-Graw-Hill, New York, USA.
- MRKVA M. (1983). Evaluation of correlations between absorbance at 254 nm and COD of river waters. Water Res., 17, 231-235.
- NIREL P.M.V. and R. REVACLIER (2003). Authentication of a physico-chemical variability index in freshwaters: relationship with biological quality. Aquat. Ecosys. Health Manage., 6, 1-5.
- PEDERSEN B., K. GERNAEY, M. HENZE and P.A. VANROLLEGHEM (2002). Evaluation for an ASM1 model calibration procedure on a municipal-industrial wastewater treatment plant. J. Hydroinform., 4, 15-38.
- ROBERTS D.J., D. NICA, G. ZUO and J.L. DAVIS (2002). Quantifying microbially induced deterioration of concrete: initial studies. Int. Biodeter. Biodegrad., 49, 227-234.
- SCHILPEROORT R.P.S., G. GRUBER, C.M.L. FLAMINK, F.H.L.R. CLEMENS and J.H.J.M. VAN DER GRAF (2006). Temperature and conductivity as control parameters for pollution-based real-time control. 5th IWA World Congress, Beijing, China,10-14 September 2006.
- THOMAS O., F. THERAULAZ, M. DOMEIZEL and C. MASSIANI (1993). UV spectral deconvolution: a valuable tool for wastewater quality monitoring. Environ. Technol., 14, 1187-1192.
- UDERT K.M, T.A. LARSEN and W. GUJER (2003a). Estimating the precipitation potential in urine-collecting systems. Water Res., 37, 2667-2677.
- UDERT K.M., T.A. LARSEN, M. BIEBOW and W. GUJER (2003b). Urea hydrolysis and precipitation dynamics in a urine-collecting system. Water Res., 37, 2571–2582.
- WU J., M.N. PONS and O. POTIER (2006). Wastewater fingerprinting by UV-visible and synchronous fluorescence spectroscopy, Water Sci. Technol., 53, 449-456.
Liste des figures
Figure 1
Experimental set-up at the Nancy-Maxéville wastewater treatment plant.
Site expérimental de la station d’épuration de Nancy-Maxévill.
Figure 2
Correlation between absorbance at 254 nm (A254) and COD.
Corrélation entre l’absorbance a 254 nm (A254) et la DCO.
Figure 3
Examples of variations of conductivity (—) and pH (---) for three different communities in summer: (a) Nancy; (b) Colombey-les-Belles and (c) Villey-Saint-Etienne.
Exemples de variations de la conductivité (—) et du pH (---) pour trois sites expérimentaux en été; (a) Nancy; (b) Colombey-les-Belles and (c) Villey-Saint-Etienne.
(a)
(b)
(c)
Figure 4
Variations of the concentrations (open symbols) of some anions (chlorides and nitrates) and cations (sodium, potassium, magnesium and calcium) in Nancy (winter season). (- - - ) A254: (a) Ca; (b) Chlorides; (c) K; (d) Mg; (e) Na; (f) Nitrates.
Variations des concentrations (symboles ouverts) de quelques anions (chlorures ry nitrates) et cations (sodium, potassium, magnésium et calcium) a Nancy (en hiver). (- - - ) A254. (a) Ca; (b) Chlorides; (c) K; (d) Mg; (e) Na; (f) Nitrates.
(a)
(b)
(c)
(d)
(e)
(f)
Figure 5
Comparison of measured (- - - ) and estimated (—) conductivities for Villey-Saint-Etienne.
Comparaison des conductivités mesurées (- - - ) et estimées (—) pour Villey-Saint-Etienne.
Figure 6
Calcium, potassium, magnesium and sodium: (a), (b), (c), (d) in Colombey-les-Belles; (e), (f), (g), (h) in Villey-Saint-Etienne.
Calcium, potassium, magnésium et sodium : (a), (b), (c), (d) in Colombey-les-Belles; (e), (f), (g), (h) in Villey-Saint-Etienne.
(a)
(b)
(c)
(d)
(e)
(f)
(g)
(h)
Figure 7
Long-range monitoring of settled wastewater in Nancy. Potassium (- - -), calcium (—) and conductivity (thick line).
Suivi sur une longue période de l’eau usée aprcs décantation primaire a Nancy. Potassium (- - -), calcium (—) et conductivité (trait épais).
Liste des tableaux
Table 1
Drinking water characteristics (average concentrations).
Caractéristiques des eaux potables (concentrations moyennes).
Location |
Na (mg/L) |
K (mg/L) |
Mg (mg/L) |
Ca (mg/L) |
Cl- (mg/L) |
SO42- (mg/L) |
|---|---|---|---|---|---|---|
Nancy |
12 |
2.5 |
11 |
21 |
20 |
97 |
Pont-a-Mousson |
5.6 |
4.9 |
15 |
83 |
- |
- |
Villey-Saint-Etienne |
5.7 |
3.6 |
12 |
108 |
- |
- |
Colombey-les-Belles |
5 |
1.5 |
4 |
130 |
- |
- |
Table 2
Comparison of urine and wastewater composition.
Comparaison des compositions de l’urine et des eaux usées
|
Litterature data (UDERT et al. 2003a, 2003b) |
ηurine |
ηwastewater |
||
|---|---|---|---|---|---|
|
Concentration (mg/L) |
CV% |
Range (mg/L) |
||
NH4+ (mgN/L) |
480 |
29 |
|
|
|
Urea (mgN/L) |
7700 |
20 |
|
|
|
NH4+hyd(mgN/L) |
8180 |
|
|
|
|
Phosphate (mgP/L) |
740 |
14 |
|
0.09 |
0.33 |
Calcium (mg/L) |
190 |
|
|
0.02 |
3.3 |
Sodium (mg/L) |
2800 |
|
1800‑5800 |
0.34 |
2.8 |
Potassium (mg/L) |
2200 |
|
1300‑3100 |
0.27 |
0.4 |
Magnesium (mg/L) |
100 |
21 |
|
0.01 |
0.3 |
Chloride (mg/L) |
3800 |
|
2300‑7700 |
0.46 |
3.8 |
Sulphate (mg/L) |
1500 |
29 |
|
0.18 |
3.3 |
Table 3
Range of variations of conductivity and concentrations in calcium, potassium, sodium and magnesium at the four sampling sites.
Domaine de variations de la conductivité et des concentrations en calcium, potassium, sodium et magnésium pour les quatre sites expérimentaux.
|
Conductivity (µS/cm) |
Calcium (mg/L) |
Sodium (mg/L) |
Magnesium (mg/L) |
Potassium (mg/L) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Min |
Max |
Min |
Max |
Min |
Max |
Min |
Max |
Min |
Max |
Nancy |
750 |
1450 |
65 |
101 |
51 |
217 |
7 |
9 |
7 |
13 |
Pont-a-Mousson |
190 |
1860 |
33 |
136 |
19 |
194 |
2 |
23 |
4.7 |
67 |
Villey-Saint-Etienne |
680 |
2840 |
56 |
306 |
41 |
457 |
9.2 |
30 |
12 |
59 |
Colombey-les-Belles |
900 |
2380 |
23 |
173 |
45 |
261 |
4 |
9 |
10 |
30 |
Table 4
Contributions of the various ions to total conductivity.
Contributions des différents ions à la conductivité totale.
Ion |
λ (mS.m2/mol) |
z |
Average concentration (mol/m3) |
Average conductivity by element (µS/cm) |
Contribution to total conductivity (%) |
|---|---|---|---|---|---|
Cl- |
7.63 |
1 |
3.78 |
28.8 |
25.9 |
Ca2+ |
5.95 |
2 |
2.07 |
24.6 |
22.1 |
Na+ |
5.01 |
1 |
3.09 |
15.5 |
13.9 |
SO42- |
8 |
2 |
8.54 10‑1 |
13.7 |
12.3 |
NH4+ |
7.34 |
1 |
1.8 |
13.2 |
11.9 |
PO43- |
9.28 |
3 |
2.68 10‑1 |
7.5 |
6.7 |
Mg2+ |
5.31 |
2 |
3.29 10‑1 |
3.5 |
3.1 |
K+ |
7.35 |
1 |
0.26 |
1.9 |
1.7 |
Total |
|
|
|
|
97.6 |
Table 5
Groundwater characteristics near the four sampling sites. Data from the Rhin-Meuse Basin Water Board. Coefficient of Variation (CV) calculated on 35 data points for Francheville and Colombey-les-Belles (except for Mg: 33 data points), 6 data points for Tomblaine and 18 data points for Loisy over five years (2000 to 2005).
Caractéristiques des eaux souterraines prcs des quatre sites expérimentaux Données de l’Agence de l’Eau Rhin-Meuse. Coefficient de variation (CV) calculés sur 35 données pour Francheville et Colombey-les-Belles (sauf Mg: 33 données), 6 données pour Tomblaine et 18 données pour Loisy sur cinq ans (2000 to 2005).
|
Francheville (5 km from Villey-Saint-Etienne) (Dogger limestone) |
Colombey-les-Belles (Dogger limestone) |
Tomblaine (Greater Nancy) (Lower Trias sandstone) |
Loisy (5 km from Pont-a-Mousson) (Moselle alluvium) |
||||
|---|---|---|---|---|---|---|---|---|
Depth |
65 m |
0 m |
708 m |
8 m |
||||
|
Average |
CV (%) |
Average |
CV (%) |
Average |
CV (%) |
Average |
CV (%) |
Conductivity (µS/cm) |
638 |
15 |
468 |
20 |
1733 |
10 |
946 |
20 |
Potassium (mg/L) |
4.6 |
16 |
0.92 |
34 |
14.7 |
6.4 |
2 |
100 |
Magnesium (mg/L) |
26 |
12 |
4.8 |
100 |
21 |
10 |
10 |
17 |
Calcium (mg/L) |
68 |
8 |
102 |
16 |
84 |
10 |
135 |
12 |
Sodium (mg/L) |
56 |
9 |
6 |
50 |
246 |
18 |
44 |
37 |