Résumés
Abstract
In North America, the potential of the American hoverfly Eupeodes americanus (Wiedemann, 1830) (Diptera: Syrphidae) as a biocontrol agent has been demonstrated, particularly against the foxglove aphid Aulacorthum solani Kaltenbach, 1843 (Hemiptera: Aphididae). Since no information is available to distinguish the larval instars of this species, the present study used the semi-continuous observation (time-lapse photography and stereo microscope) of the three larval stages to build a table of morphological traits and a dichotomic key for discriminating the larval instars by observation under stereo microscope. Discriminating traits are black hairs at first instar and fused posterior breathing tubes at third instar.
Keywords:
- black hairs,
- moult,
- posterior breathing tubes,
- time-lapse photography,
- biocontrol
Résumé
En Amérique du Nord, le potentiel du syrphe d’Amérique Eupeodes americanus (Wiedemann, 1830) (Diptera: Syrphidae) comme agent de lutte biologique a été démontré, notamment contre le puceron de la digitale Aulacorthum solani Kaltenbach, 1843 (Hemiptera: Aphididae). Aucune information n’étant disponible pour distinguer macroscopiquement les stades larvaires de cette espèce, la présente étude a utilisé l’observation semi-continue (photographie time-lapse et observation à la loupe binoculaire) des trois stades larvaires pour construire un tableau de traits morphologiques et une clé dichotomique pour discriminer les stades larvaires par observation à la loupe binoculaire. Les traits discriminants sont la présence de poils noirs au premier stade et la fusion des tubes respiratoires postérieurs au troisième stade.
Mots-clés :
- poils noirs,
- mue,
- tubes respiratoires postérieurs,
- photographie time-lapse,
- lutte biologique
Corps de l’article
Hoverflies (Diptera: Syrphidae) have proven to play an important role in the ecosystem due to their dual services as pollinators (adult) and biological control agents (larvae) (Dunn et al. 2020). Since 2014, works from the Biocontrol laboratory of University of Quebec in Montreal (UQAM) on the American hoverfly, Eupeodes americanus (Wiedemann, 1830) (Diptera: Syrphidae), have shown that this species is able to be active at low temperatures (12-14-18 °C), e.g., for: (i) flight, (ii) oviposition, and (iii) feeding activities (Bellefeuille et al. 2017, 2019). Due to those characteristics, this species shows great potential as a biological agent, even when the temperature is low. Eupeodes americanus can feed on more than 25 aphid species (Rojo et al. 2003; Vockeroth 1992), including several major pests in Quebec greenhouses such as the green peach aphid Myzus persicae Sulzer, 1776 (Hemiptera: Aphididae), the foxglove aphid, Aulacorthum solani Kaltenbach, 1843 (Hemiptera: Aphididae), the pea aphid Acyrthosiphon pisum Harris, 1776 (Hemiptera: Aphididae) and the melon aphid Aphis gossypii Glover, 1877 (Hemiptera: Aphididae).
While E. americanus appears to be a promising biological control agent, many biological traits remain to be explored such as its voracity, development, and hibernation habits. Regarding their morphological traits, only adults are well described, notably with identification keys (Skevington et al. 2009; Vockeroth 1992). However, for most hoverfly species, larval instars have not been differentiated in most of the earlier works. According to Joshi and Ballal (2013) and Rotheray and Gilbert (2011), this is probably due to the fact that exuviae are not easily visible by being very thin, transparent, and often crumpled and damaged. In Europe, the morphological description of syrphid larvae is more advanced with notably a key for the third instar larvae of most European genera (Láska et al. 2013) and a colour guide (Rotheray 1993). Some studies tend to describe all three larval instars, but they often only provide information on variable traits such as colour and body length (Bergh and Short 2008; Davidson 1919). The full development of the posterior breathing tubes is usually a fundamental criterion to identify the third instar larvae of Syrphidae (Hartley 1961; Rotheray and Gilbert 2011), but it has not been confirmed for E. americanus. Ouattara et al. (2022) showed that, like most hoverflies (Rotheray 1993; Skevington et al. 2019), E. americanus has three larval instars. However, the identification of the larval instars through morphological characters remains incomplete. This lack of knowledge is a barrier to further studies on this species. Notably, identification of instars is necessary to evaluate the syrphid’s development or voracity since aspects like development time, mortality, or number of preys consumed vary between instars. This study aims to provide a dichotomous key of discriminating morphological traits to differentiate the three larval instars of E. americanus.
Insect rearing. Insect rearing was carried out at UQAM in the Biocontrol laboratory. The melon aphids, A. gossypii, were reared on cucumber, Cucumis sativus L. (Cucurbitales: Cucurbitaceae), the bird cherry-oat aphid, Rhopalosiphum padi (Linnaeus, 1758), on barley plants, Hordeum vulgare L. (Poales: Poaceae), and the pea aphid, A. pisum, on broad bean plants Vicia faba L. (Fabales: Fabaceae) (25 °C, 16L:8D photoperiod, and 60% RH). American hoverfly, Eupeodes americanus, rearing was done as described in Bellefeuille et al. (2019). Adults were fed with pollen and sugared water and larvae were fed with R. padi reared on barley.
Photography methods for first to second instar differentiation. Eleven larvae were observed from egg hatch to the second instar (during approximately three days) using a time-lapse photography technique to visualize moulting, usually difficult to observe. Eggs were allowed to hatch on a broad bean leaf placed on humid cotton in a 5 cm diameter opened Petri dish. After hatching, larvae were transferred to cucumber or barley leaves placed on agar gel in the same type of opened Petri dish (21 °C, 9L:15D photoperiod, and 45% RH). Polytetrafluoroethylene insect barrier, PTFE Plus (Formica®) was used to prevent larvae from escaping. Larvae were fed ad libitum either with melon aphids or bird cherry-oat aphids. The Petri dish was kept under ambient light during the day and under an LED lightbulb (120 volts, 150 mA, Luminus® PLYB1305D) in a basic reflector lamp with a red filter that was continuously on at night. ISO and exposition time were the same during day and night (ISO-400 and ¼ second exposition time). Time-lapse was set at a 5 min delay between each shot and a Canon EOS 50D® with a 4x optical zoom was used. The camera was placed at approximately 65 cm above the Petri dish. Photos were observed twice a day. The moulting was identified by the behaviour of the larva (standing still for a moment and then stepping out of its exoskeleton, leaving it on the leaf) and by observing the exuviae using a binocular microscope. Less than 24 h after moulting (after confirming that no other moult took place between first moult and observation), larvae were observed under a binocular microscope. A preliminary descriptive grid from the first to the second instar was created assessing principal morphological traits for each instar. ImageJ (an open-source image processing program developed by the National Institutes of Health, Bethesda, Maryland, USA) software was used to measure the length of all larvae less than 10 min after hatching or moulting (n = 13 for the first instar and n = 11 for the second instar).
Observation method for second to third instar differentiation. To confirm if the finding of Rotheray and Gilbert (2011) could be applied to E. americanus third instar larvae, ten new second instar larvae were placed individually in Petri dishes and fed with A. pisum (24 °C, 16L:8D photoperiod and 40% RH). Time-lapse photography could not be used since third instar larvae were too mobile. Larvae were observed twice a day under a stereo microscope until moult. The posterior breathing tubes development was described. Moult of larvae was determined by observation of the exuviae. Less than 24 h after moulting, larvae were measured using ImageJ. Thanks to this experiment, the descriptive grid was completed up to the third instar.
Confirmation of E. americanus larval instars descriptive grid. The grid was then confirmed with the daily observation of 13 larvae from 24 h after hatching until the third instar, reared in controlled conditions (25 °C, 16L:8D photoperiod and 60% RH), and given ad libitumA. gossypii and R. padi aphids.
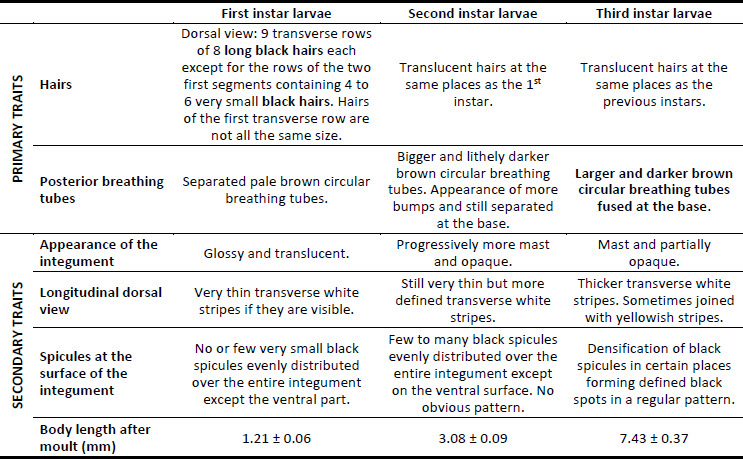
Results allowed the determination of primary and secondary traits (Table 1). Primary traits are constant over time and among individuals and are used to discriminate the larval instars of E. americanus. Secondary traits are more descriptive and may vary significantly over time (at the same instar) or according to the individual.
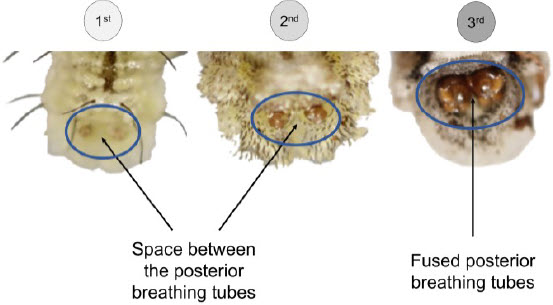
Two primary traits have been established. The colour of the dorsal hairs serves to differentiate the first instar from the two others. Noticeably, only the first instar has long black hairs which become translucent in the second and third instars (Fig. 1). The fusion of posterior breathing tubes, forming two contiguous circles, is the main trait that discriminates the third from the other instars. In fact, posterior breathing tubes are clearly spaced from each other in the first and second instars (Fig. 2).
Several secondary traits differ between the three instars (Fig. 3). For example, the number of spicules on the surface of the integument increases and gradually forms a pattern (forming dense groups of spicules distributed regularly among the dorsal surface of the integument). The thickness of the white stripes visible on the dorsal part of the larva also gradually increases over time. The stripes of the third instar larvae can be up to four times as wide as the stripes of the first instar larvae. The appearance of the integument also changes during development, going from glossy and translucent to mast and partially opaque. Finally, the length of the larvae can be used as a general indicator of the instar but varies greatly among individuals and depending on rearing conditions. Less than 24 h after hatching or moulting, first, second, and third instar larvae measure respectively about 1.23 ± 0.06 mm 3.08 ± 0.09 mm, and 7.43 ± 0.37 mm.
The present results allow to clearly discriminate the three larval instars of the American hoverfly, by using morphological traits. Even if it was not used as a discriminatory character, the change in the colour of hairs from the first to the second instar was previously described with Eupeodes luniger (Meigen, 1822) (Diptera: Syrphidae), by Bhatia (1939) and uncoloured hairs were also reported for species of the genus Scaeva Fabricius, 1805 (Diptera: Syrphidae) by Láska et al. (2006). The fused breathing tubes at the third instar were also observed in numerous species (Bhatia 1939; Hartley 1961). Other secondary characters from our descriptive grid were also reported in the literature. The presence of nine transverse rows of hairs at all stages was ubiquitous among the seven Nearctic, Palearctic and Holarctic species studied by Bhatia (1939). The appearance of the integument transi-tioning from transparent to opaque among stages as well as the presence of spinules or spicules at the third stage was also reported in numerous species (Bhatia 1939; Hartley 1961; Láska et al. 2006). Nonetheless, variation in those traits is evident as Bhatia (1939) showed that Episyrphus balteatus De Geer, 1776 (Diptera: Syrphidae) and Sphaerophoria rueppellii (Wiedemann, 1830) (Diptera: Syrphidae) integuments were transparent and shiny even at the third instar.
Table 1
Morphological traits grid for discrimination of the three larval instars of E. americanus
The primary traits allow the differentiation of the three larval instars by observation under the stereo microscope. The secondary traits are additional information and may vary slightly depending on the individual.
The description of these primary and secondary traits of E. americanus larvae broadens the morphological knowledge on each instar of this species and makes it possible to easily differentiate them, either in the laboratory or in the field, with a hand lens. Being a promising biological control agent, it is important to assess fundamental characteristics for its utilization like its voracity, larval development, and mortality rate. Since these characters differ between the instars, it is essential to differentiate them. It is also useful for optimizing the mass rearing of E. americanus. Moreover, time-lapse photography has proven to be a useful technique for monitoring the larval development of hoverflies. It allows knowing exactly when the moulting process takes place without having to find the exuviae which can be very difficult to see otherwise (Joshi and Ballal 2013; Rotheray and Gilbert 2011). Thanks to the high definition of the pictures, time-lapse photography allows observing details on a very small scale while requiring very little data storage compared to video. This little-used method would benefit from being exploited more.
Figure 1
Dichotomous key for larval instars identification of E. americanus
Figure 2
Detailed pictures of the posterior breathing tubes of E. americanus larvae
Figure 3
Secondary morphological traits of E. americanus three larval instars
We thank Mathieu Lemieux for the technical help regarding the photography. We also thank the entire team from the Laboratoire de lutte biologique and give a special thank to Catherine Thouin for the measurements of the larvae. Finally, we thank Graham Rotheray for his help in identifying integumental structures of E. americanus. This study was funded by the Ministère de l’Agriculture, des Pêcheries et de l’Alimentation du Québec (MAPAQ), following the program Prime-Vert 18-009-UQAM and by a CRSNG discovery grant to Eric Lucas. Funding was also provided by Agriculture and Agri-Food Canada through the Canadian Agricultural Partnership, under the AgriScience Program.
Parties annexes
REFERENCES
- Bellefeuille, Y., M. Fournier, and E. Lucas. 2017. Eupeodes americanus and Leucopis annulipes as potential biocontrol agents of the foxglove aphid at low temperatures. IOBC-WPRS Bull. 124: 62-66.
- Bellefeuille, Y., M. Fournier, and E. Lucas. 2019. Evaluation of two potential biological control agents against the foxglove aphid at low temperatures. J. Insect Sci. 19: Article 2.
- Bergh, J.C., and B.D. Short. 2008. Ecological and life-history notes on syrphid predators of woolly apple aphid in Virginia, with emphasis on Heringia calcarata. BioControl 53: 773-786.
- Bhatia, M.L. 1939. Biology, morphology and anatomy of aphidophagous syrphid larvae. Parasitology 31: 78‑120.
- Davidson, W.M. 1919. Notes on Allograpta fracta O. S. (Diptera: Syrphidae). Can. Entomol. 51: 235-239. doi:10.4039/ent 51235-10
- Dunn, L., M. Lequerica, C.R. Reid, and T. Latty. 2020. Dual ecosystem services of syrphid flies (Diptera: Syrphidae): pollinators and biological control agents. Pest Manag. Sc. 76: 1973-1979.
- Hartley, J.C. 1961. A taxonomic account of the larvae of some British syrphidae. Proc. Zool. Soc. Lond. 136: 505-573.
- Joshi, S., and C.R. Ballal. 2013. Syrphid predators for biological control of aphids. J. Biol. Control. 27: 151-170.
- Láska, P., L. Mazánek, and V. Bičík. 2013. Key to adults and larvae of the genera of European Syrphinae (Diptera, Syrphidae). Acta Mus. Sil., Sci. Nat. 62: 193-206.
- Láska, P., C. Pérez-Bañón, L. Mazánek, S. Rojo, G. Ståhls, M.A. Marcos-García, V. Bičík, and J. Dušek. 2006. Taxonomy of the genera Scaeva, Simosyrphus and Ischiodon (Diptera: Syrphidae): descriptions of immature stages and status of taxa. Eur. J. Entomol. 103: 637-655.
- Ouattara, T.Y., M. Fournier, S. Rojo, and E. Lucas. 2022. Development cycle of a potential biocontrol agent: the American hoverfly, Eupeodes americanus, and comparison with the commercial biocontrol agent Aphidoletes aphidimyza. Entomol. Exp. Appl. 170: 394-401.
- Rojo, S., F. Gilbert, M.A. Marcos-Garcia, J.M. Nieto, and M.P. Mier Durante. 2003. A world review of predatory hoverflies (Diptera, Syrphidae: Syrphinae) and their prey. Centro Iberoamericano de la Biodiversidad, Alicante, Spain. 219 pp.
- Rotheray, G.E. 1993. Colour guide to hoverfly larvae (Diptera, Syrphidae) in Britain and Europe. Derek Whiteley, Sheffield, England. 156 pp.
- Rotheray, G.E., and F. Gilbert. 2011. The natural history of hoverflies. Forrest Text, Tresaith, United Kingdom. 333 pp.
- Skevington, J.H., M.M. Locke, A.D. Young, K. Moran, W.J. Crins, and S.A. Marshall. 2019. Field guide to the flower flies of Northeastern North America. Princeton University Press, Princeton, NJ, USA. 512 pp.
- Vockeroth, J.R. 1992. The flower flies of the subfamily Syrphinae of Canada, Alaska, and Greenland (Diptera: Syrphidae). Agriculture Canada, Ottawa, Canada. 455 pp.
Liste des figures
Figure 1
Dichotomous key for larval instars identification of E. americanus
Figure 2
Detailed pictures of the posterior breathing tubes of E. americanus larvae
Figure 3
Secondary morphological traits of E. americanus three larval instars
Liste des tableaux
Table 1
Morphological traits grid for discrimination of the three larval instars of E. americanus