Résumés
Abstract
Metabolomics is one of the most eminent and newly emerging omic sciences. It is a powerful tool to study metabolic changes that occur in an organism. Plants produce a wide range of metabolites and the study of these metabolites can answer a number of questions that arise in the minds of researchers. Change in the metabolites is the most important feature in a genetically modified plant or plant interactions with pests, pathogens and the environment. Plant pathogen interactions are amongst the most biochemically complex mechanisms and pose a great challenge in front of plant pathologists; metabolomics not only play a great role in deciphering these complex interactions but also the study of certain defence-related metabolic changes can be utilized in a number of ways to protect the plant from the harmful pathogens. The science of metabolomics utilizes a number of techniques to study the wide variety of metabolites. This review will give a brief about the various techniques used in metabolomics and how some of these techniques have been successfully utilized in the field of plant pathology.
Keywords:
- metabolites,
- metabolomics,
- pathogens,
- plants
Résumé
La métabolomique est l’une des sciences omiques les plus éminentes et les plus récentes. Elle constitue un outil puissant pour étudier les changements métaboliques qui se produisent dans un organisme. Les plantes produisent un large éventail de métabolites et l’étude de ces métabolites peut répondre à un certain nombre de questions que se posent les chercheurs. La modification des métabolites est le caractère déterminant dans une plante génétiquement modifiée ou dans les interactions de la plante avec les ravageurs, les pathogènes et l’environnement. Les interactions entre les plantes et les pathogènes sont parmi les mécanismes les plus complexes sur le plan biochimique et constituent un grand défi pour les phytopathologistes; la métabolomique joue non seulement un rôle important dans le décryptage de ces interactions complexes, mais l’étude de certains changements métaboliques liés à la défense peut être utilisée de plusieurs façons pour protéger la plante contre les pathogènes nuisibles. La science de la métabolomique utilise un certain nombre de techniques pour étudier une grande variété de métabolites. Cette revue donne un aperçu des différentes techniques utilisées en métabolomique et comment certaines de ces techniques ont été utilisées avec succès dans le domaine de la phytopathologie.
Mots-clés :
- métabolites,
- métabolomique,
- pathogènes,
- plantes
Corps de l’article
INTRODUCTION
Plant pathogen interactions are highly complex in nature, as pathogens and plants try to counter each other. Plants in their defence to pathogens produce a wide range of secondary metabolites. Plants carry out a web of different bio-synthetic pathways for the production of these metabolites. Since metabolites are the final products of all the cellular processes, the levels of these metabolites can give an idea regarding the ultimate response of biological systems to any kind of genetic or environmental changes (Fiehn 2002). Metabolomics is a newly emerging omic science which facilitates us to do the quantitative and qualitative analysis of these metabolites. This can help us to get acquainted with the actual biological interactions taking place in a particular biological system. Metabolites are highly dynamic that is they keep on changing with time and space and the versatility of these metabolites in terms of structure and function makes their analysis a bit difficult task (Stitt and Fernie 2003). Study of the complete metabolome through a single technique is impossible as all the metabolites differ in certain properties, therefore various metabolomic techniques are utilized like mass spectrometry (MS) based methods including GC-MS (gas chromatography-MS), LC-MS (liquid chromatography-MS), CE-MS (capillary electrophoresis-MS), and FI-ICR-MS (Fourier transform ion cyclotron resonance-MS), and non-destructive NMR (nuclear magnetic resonance spectroscopy) (Khakimov et al. 2014; Okazaki and Saito 2012). Looking into the complexities of the study, this review aims at providing a brief about the different techniques that can be used to study the metabolic changes that occur during plant pathogen interactions, also will focus on some examples of how these techniques are being utilized by the researchers in deciphering the changes in metabolic profile of plants.
Metabolomics
Omic sciences are gaining a lot of popularity nowadays and the advancements in the technologies and informatics used to generate and process large sets of biological data are promoting a critical shift in the study of biological sciences. Omic sciences mainly aim at the universal detection of genes (genomics), mRNA (transcriptomics), proteins (proteomics) and metabolites (metabolomics) in a specific biological sample in a non-targeted and unbiased manner (Horgan and Kenny 2011). The integration of all these techniques can more appropriately be termed as “system biology”. Omic science not only aids us in better understanding of normal physiological processes but also can be efficiently utilized to study the disease processes through screening, diagnosis and prognosis as well as to perceive the etiology of diseases (Horgan and Kenny 2011). Metabolomics is a branch of omic sciences and it can be defined as the study of global metabolite profiles in a system (cell, tissue or organism) under a given set of conditions (Goodacre et al. 2004). Metabolomics is said to have many theoretical advantages over the other omic technologies. The metabolome is considered as the final output of transcription of a gene and, thus, metabolic changes are amplified compared to the changes in the transcriptome and the proteome (Urbanczyk et al. 2003). Moreover as the down-stream product, the metabolome is more closely associated to the phenotype of the biological system (Burt and Nandal 2016). Though amongst all the omic sciences metabolome forms the smallest domain (~5000 metabolites), but due to the presence of a wide variety of metabolites it is physically and chemically more complex compared to other omics.
Metabolic changes during plant pathogen interactions
Plants unlike animals do not have an immune system but it’s not that they cannot defend themselves against the attack of harmful organisms or adverse environmental conditions. They utilize a unique network of metabolic processes which leads to the production of certain miraculous compounds (plant defence proteins and enzymes): secondary metabolites. These secondary metabolites are not directly involved in growth, development and reproduction of an organism (as are primary metabolites) but are known to play certain other specific roles (Agostini-Costa et al. 2012). The huge plant community is known to produce more than 100 000 secondary metabolites which are categorized under certain taxonomic groups (Oksman-Caldentey and Inzé 2004). They can be either preformed metabolites also referred as phytoanticipins which get converted into toxic molecules upon pathogen perception or phytoalexins that are produced after the perception of pathogens (Arbona and Gómez-Cadenas 2016). As in mulberry plant infection with fungal pathogens Fusarium solani or Stigmina mori led to the identification of a diphenyl-propane derivative named morusin which on accumulation is inhibitory to fungal and bacterial growth (Gottstein and Gross 1992).
These secondary metabolites are produced by different synthetic pathways and on the basis of that are broadly classified into three: terpenes, phenolic compounds and nitrogen-containing compounds (Fang et al. 2011). The study of these metabolites can be utilized as a tool to decipher plant pathogen interaction and also to differentiate the type of compounds formed in resistant and susceptible reactions. Pushpa et al. (2014) conducted a non-targeted metabolic study of resistant and susceptible potato cultivars (F06037 and Shepody respectively) against Phytophthora infestans (US-8 genotype) using LC-MS. It was observed that hydroxy-cinnamic acid amides (HCAAs) of the shunt phenylpropanoid pathway are increased in resistant cultivars after inoculation with pathogens. Deposition of HCAAs in the host cell wall inhibits pathogen colonization. Various flavonoids have been extensively studied in plant defence: pisatin in pea (Perrin and Bottomley 1961), medicarpin in alfalfa (He and Dixon 2000), glyceollins in soybean (Ebel et al. 1976) and many others. They are not only antifungal but also antibacterial thus providing resistance against a wide range of pathogens (Piasecka et al. 2015). Plant metabolites are not only altered by fungal and bacterial pathogens but viral diseases also interfere with plant metabolism. In order to facilitate its own dispersal Cucumber Mosaic Virus (CMV) leads to emission of certain volatile compounds from the infected squash to attract the aphid vectors (Mauck et al. 2014). Similarly, tobacco which is a relatively poor host for Bemisia tabaci (Gennadius) on infection with Tomato yellow leaf curl virus (TYLCV) shows some alteration in synthesis of volatile terpenoid and increases host suitability (Luan et al. 2013). All these alterations in and through metabolites during plant pathogen interactions increase our curiosity on what the particular compound is involved and what are the techniques that we can use to identify them, so the basic steps for the study of metabolites are as follows.
Basic steps in metabolomics (Courant et al. 2014)
Sample preparation
Metabolites differ in their physicochemical properties thus sample collection and preparation will depend on the kind of metabolite to be studied and the method used for processing and detecting those metabolites. In some cases preliminary quenching is needed to stabilize the sample which stops the metabolic reactions (Álvarez-Sánchez et al. 2010). It is recommended to store sample at -80 °C and sample aliquoting should be done at the time of collection to avoid repetitive freezing and thawing of samples (Dunn et al. 2011).
Metabolomic profiles generation
Due to great diversity in the metabolites there is no single technique for the separation and quantification for all the metabolites. A number of techniques are used for metabolites with different properties. GC-MS is mostly used for volatile compounds while analysis of polar or ionic metabolites may be achieved with LC-MS. CE-MS can also be used for polar or ionic compounds (Barbas et al. 2011).
Data processing
Metabolomic studies produce a huge amount of raw data. It is really difficult to handle such complex datasets manually and thus specific software tools and algorithms are required to convert this complex raw data to comprehensive extracted data that can easily be processed using statistical tools (Courant et al. 2014).
Data analysis
Metabolomics generate high dimensionality data. In metabo-lomic fingerprinting, the number of variables measured per subject vastly exceeds the number of subjects to be considered under that study (Guo et al. 2010). Univariate methods such as the classical Student’s t-test can be used to identify candidate compounds showing significant differences in levels between two subgroups of samples. Multivariate techniques are also used to reduce the complexity of the datasets and to derive the analytical information of biological importance (Brown et al. 2005; Trygg et al. 2007).
Techniques used in metabolomics
Metabolomics deal with two types of analysis: targeted and non-targeted analysis of both endogenous as well as exogenous metabolites (< 1500 Da). Metabolites may include peptides, amino acids, nucleic acids, organic acids, carbohydrates, vitamins, alkaloids, polyphenols and other inorganic compounds (Sumner et al. 2003). These metabolites can also act as biomarkers (Arakaki et al. 2008). Metabolomics has been applied to define metabolites related to prognosis or diagnosis of diseases and could provide greater pathophysiological understanding of disease (Zhang et al. 2012). Metabolome being a very complex entity cannot be analyzed by a single analytical approach thus a combination of various novel techniques such as Gas Chromatography (GC), High Performance Liquid Chromatography (HPLC), Ultra Performance Liquid Chromatography (UPLC), Capillary Electrophoresis (CE) is needed for the separation of the metabolites based on different properties of the compounds (Hegeman 2010). These separation techniques need to be coupled to detection techniques such as Mass Spectrometry (MS) and Nuclear Magnetic Resonance (NMR) spectroscopy. Here is a brief description about the principle on which these different techniques are based and their application in the field of plant pathology:
Liquid Chromatography Mass Spectrometry (LC-MS)
The advance form of liquid chromatography includes HPLC and UPLC. In HPLC and UPLC, liquid is the mobile phase. Both the technique work on the same principle that is the molecules passing through the stationary phase moves in different speeds depending on their chemical structure. Though complex biological mixtures cannot be separated through LC columns (Tolstikov and Fiehn 2002) but it allows good mass library reproducibility (Huhman and Sumner 2002). HPLC separations are widely used for analysis of labile and non-volatile polar and non-polar compounds in their native form. UPLC utilizes porous particles with diameters smaller than 2 mm leading to increased surface area and thus provide a better separation. UPLC has certain advantages over HPLC as it allows analysis of more number of samples in a particular time, has high efficacy and peak capacity compared to that of HPLC columns (Zhang et al. 2012).
Alterations in the metabolites of three South African sorghum cultivars; namely Amazi Mhlophe, NS 5511 (bitter abbreviated B) and NS 5655 (sweet, abbreviated S) responding to Colletotrichum sublineolum, were investigated by Tugizimana et al. (2019) using LC-MS based untargeted metabolomics and accumulation of a wide range of antifungal phenolic compounds were observed, particularly apigeninidin, luteolinidin, 3-deoxyanthocynidin phytoalexins and other related conjugates were detected. Yogendra et al. (2014) did a non-targeted metabolomic study of the resistant related metabolites by comparing metabolomics of potato late blight resistant (AC04 and AC09) and susceptible potato genotypes (Criolla Colombia). Trough LC-MS, it was observed that defence-related compounds such as flavonoids phenylpropanoids and alkaloid chemical groups were highly induced in resistant genotypes compared to susceptible ones. Deposition of HCAAs, alkaloids and amides flavonoids leads to the thickening of the cell wall leading to resistance against pathogen penetration.
Chalcone synthase is a key enzyme of flavonoid synthesis pathway which leads to accumulation of cell wall-bound phenolic compounds resulting in resistance in Arabidopsis plants against bacterial pathogen Pseudomonas syringae (Soylu 2006).
Chen et al. (2018) conducted untargeted metabolomics of Arabidopsis through LC-MS. The focus of the study was to observe the role of metabolite N-hydroxy-pipecolic acid in inducing systemic disease resistance in Arabidopsis against bacterial pathogen, P. syringae pathovar tomato. The study demonstrated that Flavin-Dependent Monooxygenase 1 (FMO1), which plays a key role in inducing systemic acquired resistance (SAR), can synthesize N-OH-Pip from pipecolic acid present in plant, and fmo1 mutants when applied exogenously with N-OH-Pip move systemically in Arabidopsis plant and can overcome the SAR-deficiency of the mutants. Several plant metabolites including Pipecolic acid (Pip) (Návarová et al. 2012) are reported to be involved in signal amplification and long-distance communication during SAR (Dempsey and Klessig 2012; Shah and Zeier 2013; Shah et al. 2014).
Gas Chromatography Mass Spectrometry (GC-MS)
GC-MS is mainly used for volatile compounds which vaporize without decomposition. Mobile phase is mainly an inert or unreactive gas such as nitrogen or helium. Mostly helium gas is used as carrier gas. Compounds which are volatile and thermally stable such as short chain alcohols, acid, esters, and hydrocarbons are mainly analyzed using this technique.
GC can also be used for analysis of many other compounds only after following derivatization which includes alkylation and silylation (Begley et al. 2009; Broeckling et al. 2005). Polar compounds like carbohydrates, carboxylic acids and free amino acids can be analyzed using GC-MS as derivatives of methoxime/trimethylsilyl (Roessner et al. 2001) whereas no further processing is needed for volatiles (Beck et al. 2014). The sample metabolites differ in their retention times and thus can be automatically identified by comparing and matching the retention time from mass spectra of chroma-tograms through pure chemical standards (Gross 2004).
Studies of plant metabolites can also help to differentiate pathogenic infections and thus may be a great tool for detection and diagnosis. A study was conducted by Lui et al. (2005) to discriminate three fungal diseases of potato through metabolite profiling using GC-MS. Potato tubers were inoculated with three pathogens: P. infestans, Botrytis cinerea and Pythium ultimum. Tubers inoculated with Botrytis produced two specific volatiles: 2-2-propenyl-1,3-dioxolane and 3, 5-heptadiyn-2-one and those inoculated with Pythium produced three metabolites: 2-butanone, 2-methyl-l-butanol and 2-methyl-2-butanalnine. However ethoxy-ethene was observed in tubers inoculated with Phytophthora inoculated tubers.
Several plant defence compounds have been analyzed using GC-MS techniques; various phenolic compounds were mainly found in potato (Wagner et al. 2003; Yang and Bernards 2007), soybean leaves (Benkeblia et al. 2007), maize (Röhlig et al. 2009) and tobacco (Dauwe et al. 2007).
Capillary Electrophoresis Mass Spectrometry (CE-MS)
In capillary electrophoresis, analytes are separated on the basis of their ionic mobility or partitioning into an alternate phase through non-covalent interactions. CE-MS is an effective promising separation technique providing high-analyte resolution, for charged metabolites and provides information mainly on polar or ionic compounds (Barbas et al. 2011). It applies separations with high resolutions, sensitive mass determination and specific chemical standards to identify and quantify thousands of metabolites on the basis of their charge over both negative and positive ionization modes (Sato et al. 2004). It is really applicable for the analysis of water-soluble polar metabolites.
Several metabolites, the phenylpropanoid and shikimate pathways, show rise in their levels after Rhizoctonia solani infection (Mutuku and Nose 2012). Plant defence mechanism involves a number of pathways and phenylpropanoid and shikimate pathways form an important part of plant defence (Dixon et al. 2002; Tzin and Galili 2010) and they are involved in the synthesis of various secondary metabolites including phenol compounds (Lattanzio et al. 2006). Synthesis of phenols plays a key role in providing resistance against infection caused by R. solani (Akhtar et al. 2011).
Suharti et al. (2016) studied the difference in levels of metabolites in R. solani infected resistant and susceptible lines of rice (32R and 29S, respectively) through capillary electrophoresis equipped with time of flight mass spectro-photometry (CE/TOF-MS) in positive ion mode. Chlorogenic acid metabolite showed a positive response in 32R and amino acids which showed increase in 29S after inoculation with R. solani were: γ-aminobutyric acid, glutamate, glycine, phenylalanine, histidine, tryptophan, tyrosine and serine. Several compounds produced in plants are plant defence response regulators. Mucha et al. (2019) did a CE study of systemin (an eighteen amino acid peptide plant hormone) which plays an important role in regulating plant defence response. Systemin peptide was injected into the leaves and stem of tomato plant and its transportation in the plant tissues was traced by CE. Systemin is a signalling compound which plays an important role in systemic defence; about 20 defensive genes (like proteinase inhibitors or polyphenol oxidase genes) are activated and regulated by systemin (Pearce et al. 1991; Ryan 2000).
Matrix Assisted Laser Desorption Ionisation Mass Spectrometry Imaging (MALDI-MSI)
The technique involves use of matrix-assisted laser desorption ionization as a mass spectrometry imaging technique in which the sample (tissue section) is moved in two dimensions while the mass spectrum is recorded (Chaurand et al. 2006). This method has an advantage as it measures the distribution of several analytes at one time without destroying the sample. This technique provides a means to study the plant pathogen interaction and to discover potential markers of infection. It utilizes a matrix, typically a small organic acid with strong ultraviolet absorbance, mixed with analytes to aid desorption and ionization (Norris and Caprioli 2013). The resulting gas phase analyte ions are detected and displayed in a spectrum according to their mass-to-charge ratios (m/z), which yield specific molecular signatures within complex samples. This label-free technology can be used without a priori knowledge of sample composition, allowing for the detection of a variety of analytes, from small molecules to large proteins (Angel and Caprioli 2013).
The technique is a gift of modernization in technology, and can be a great advantage in studying plant pathogen interactions. The technique was successfully utilized by Becker et al. (2014) to study the compounds produced by grapevine as a defence response against Plasmopara viticola. It was observed from the study that other than reservetrol, more toxic compounds such as pterostilbene and viniferins are produced as defence compounds. The technique plays an important role of detection of pathogenic microbes in soil (Siricord and O’Brien 2008).
Ion exchange chromatography
Ion exchange chromatography is an important technique for separation of ionic compounds. It is mainly of two types: anion exchange and cation exchange. In this technique, analyte molecules are retained on the column through ionic interactions. The ion exchange chromatography matrix consists of positively and negatively charged ions.
Elicitors are known to induce plant defence responses, such as cell wall strengthening, ethylene biosynthesis, reactive oxygen species, induction of hypersensitive response proteins, and expression of pathogenesis-related (Miyata et al. 2006; B. Wang et al. 2012; J.Y. Wang et al. 2004). Some beneficial bacteria, such as the plant growth-promoting rhizobacteria (PGPR), have the tendency to reduce the activity of pathogenic microorganisms by microbial antagonism through competing for nutrients, secretion of lytic enzymes and production of antibiotics (Handelsman and Stabb 1996; van Loon and Bakker 2003; van Loon and Glick 2004). Furthermore, they can indirectly prevent plant from pathogens by eliciting the plant defence system (Haas and Défago 2005).
Protein elicitors from some biocontrol strains have been reported to induce disease resistance, such fengycins and surfactins from Bacillus subtilis (Ongena et al. 2007). Shen et al. (2019) reported novel protein elicitor (AMEP412) from B. subtilis BU412 which leads to hypersensitive response (HR) and SAR in tobacco. Ion-exchange and size exclusion chroma-tography were used for purification. The study revealed that AMEP412 can trigger a series of defence mechanisms such as the generation of reactive oxygen species. It also induced defence enzymes, including phenylalanine ammonialyase, polyphenol oxidase, peroxidase and superoxide dismutase. AMEP412 could stimulate plant systemic resistance against P. syringae pv. tomato DC3000.
N. Wang et al. (2016) reported a novel protein elicitor from Bacillus amyloliquefaciens NC6 which was responsible for induction of systemic resistance in tobacco. The elicitor led to the HR necrosis in leaves of tobacco plant. Systemic resistance was observed against a broad range of pathogens, including tobacco mosaic virus (TMV) and B. cinerea. The elicitor up regulated many genes including the phenylalanine ammonia lyase (PAL), salicylic acid (SA)-responsive PR1a, PR1b, PR5, Coronatine insensitive 1 (COI1) and jasmonic acid (JA)-responsive PDF1.2 which play a key role in plant defence.
Certain fungi produce some antifungal compounds which can be utilized in a number of ways. The first filamentous fungus to potently produce an antifungal peptide (AFP) was Aspergillus giganteus (Olson and Goerner 1965; Wnendt et al. 1994). Rao et al. (2015) identified an antifungal protein, AfAFPR9, and purified it from supernatant of culture of Aspergillus fumigatus R9. AfAFPR9 showed antifungal effect against plant pathogenic fungi Fusarium oxysporum, Alternarialongipes, Colletotrichum gloeosporioides, Paecilomyces variotii, and Trichoderma viride at minimum inhibitory concentrations of 0.6, 0.6, 1.2, 1.2, and 2.4 μg disc-1, respectively.
Nuclear Magnetic Resonance Spectroscopy (NMR)
NMR is a new and non-destructive technique that requires minimal amount of sample preparation and has a high throughput (can process hundreds of samples per day). The principle of NMR utilizes compounds with odd atomic mass numbered nuclei, which tend to act like magnets in a provided external magnetic field through a process known as nuclear spin (Hatada and Kitayama 2004).
Plant pathogens cause metabolic perturbations in plants which can be studied through metabolite profiling of infected plants to gain insights into plant disease response (Abdel-Farid et al. 2009; Brechenmacher et al. 2010; Choi et al. 2004; Dai et al. 2010; Krishnan et al. 2005; Skirycz et al. 2010; Ward et al. 2011). Bednarek et al. (2005) used wild-type and mutant root cultures of Arabidopsis thaliana infected by root rot pathogen Pythium sylvaticum and observed how aromatic metabolite profiles differ. 1H-NMR was used to study one heterocyclic, sixteen indolic and three phenylpropanoid compounds. The study revealed a relative increase in levels of indolics upon infection. It also concluded that nature and quantity of phenylpropanoid metabolites differ in roots and leaves. Lima et al. (2010) studied the metabolic differences in the healthy and esca disease infected Vitis vinifera plants. 1D and 2D 1H-NMR was used for evaluation and it was observed that various phenolic compounds, methanol, alanine, and g-aminobutyric acid content were increased in diseased plants which may be due to plant defence activation.
Hong et al. (2012) studied the metabolic changes occurring in grapes infected by B. cinerea. Metabolite profiling was done using H-NMR and multivariate statistical analysis of berries from botrytized and healthy bunches. It was observed that phenylpropanoids, flavonoid compounds, glycerol, succinate and gluconic acid, all were directly associated with B. cinerea growth, and were only detected in Botrytis infected berries.
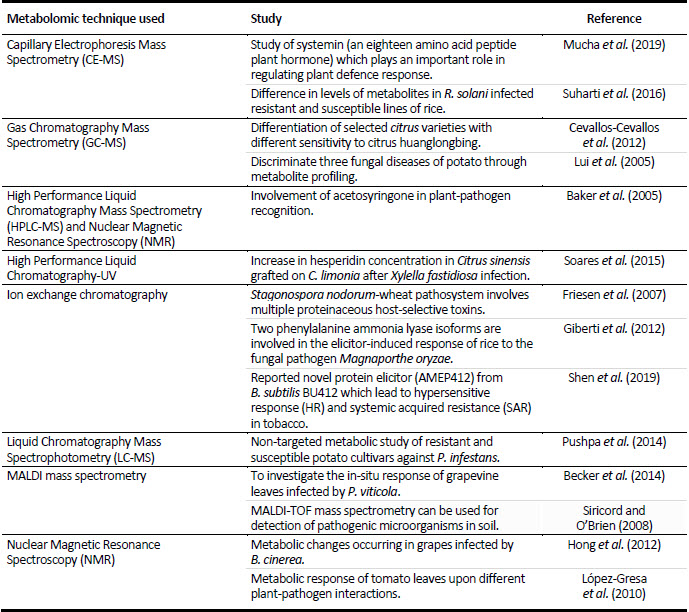
The Table 1 explains how variantly these metabolomic techniques have helped us to study the plant pathogen interactions. In case of plant disease complex, they can be utilized to differentiate the diseases, the detection of some important compounds which are produced in the defence mechanism by plants is made easier, also how resistant and susceptible varieties are different can be detected. Studying the soil microbes is difficult but the techniques make it possible for us to identify the microbes that are involved in disease causation in plants. Thus, all these techniques can be considered as the gift of modern technologies to mankind. Metabolomic tools are now being utilized in the field of plant pathology as detectives to find out how does beneficial microbes instigate the defence mechanism in plants, how does the microbiome present in its periphery plays a role in favour or in against the occurrence of a disease, how does certain chemical formulations when applied to plants lead to biochemical changes in the host plant to defend against pathogens, how does a pathogen has an audacity to breach the plant defence mechanisms, how can improved soil health leads to a microbiota which strengthens the plants against pathogens, what are the chemical talks between the microbes inside the plant system or on the surface that greatly decide the fate of the plants.
CONCLUSION and future prospects
Metabolomics has greatly helped in exploring various aspects in the field of plant pathology. It has covered a broad range right from exploring certain anti-pathogenic compounds to understanding the plant pathogen interactions and to decipher the plant defence responses against pathogens. It enables us to know novel insights of genetic, biochemical and metabolic network of cellular function (Tugizimana et al. 2013). It has also played a role in exploring various compounds from other beneficial microbes to induce resistance in plants against a wide range of pathogens. Since metabolites are more relevant to the plant phenotype advancements in their studies will be very helpful (Niederbacher et al. 2015). Different techniques have been developed to enhance the efficiency of identification and quantification of metabolites with large variation in their properties. Since metabolites have a very wide range out of which a few have been explored and there are many more to be explored to answer every new question that arrives in our minds regarding any kind of change occurring in an individual in response to certain stimuli. Development of more comprehensive data analysis software for better interpretation of metabolic studies will make it easier to draw conclusions. Developing a database or metabolite libraries can be of great use for comparing the different compounds which we obtain from plants. Though there are a number of libraries available but strengthening them would be beneficial. A combination of the knowledge in various disciplines can make it easier for us to find the solutions of many unanswered questions.
Table 1
List of some metabolomic techniques and their use in studying plant pathogen interaction
Parties annexes
REFERENCES
- Abdel-Farid, I.B., M. Jahangir, C.A.M.J.J. van den Hondel, H.K. Kim, Y.H. Choi, and R. Verpoorte. 2009. Fungal infection-induced metabolites in Brassica rapa. Plant Sci. 176: 608–615.
- Agostini-Costa, T.D.S., R.F. Vieira, H.R. Bizzo, D. Silveira, and M.A. Gimenes. 2012. Secondary metabolites. Pages 131-164 in S. Dhanarasu (ed.), Chromatography and its applications. InTech Open, Rijeka, Croatia.
- Akhtar, J., V.K. Jha, and H.C. Lal. 2011. Post-infectional phenolic changes in maize due to Rhizoctonia solani f. sp. sasakii causing banded leaf and sheath blight. Indian Phytopathol. 64: 261-264.
- Álvarez-Sánchez, B., F. Priego-Capote, and M.D. Luque de Castro. 2010. Metabolomics analysis II. Preparation of biological samples prior to detection. Trends Anal. Chem. 29: 120-127.
- Angel, P.M., and R.M. Caprioli. 2013. Matrix-assisted laser desorption ionization imaging mass spectrometry: in situ molecular mapping. Biochemistry 52: 3818-3828.
- Arakaki, A.K., J. Skolnick, and J.F. McDonald. 2008. Marker metabolites can be therapeutic targets as well. Nature 456: 443.
- Arbona,V., and A. Gómez-Cadenas. 2016. Metabolomics of disease resistance in crops. Curr. Issues Mol. Biol. 19: 13-30.
- Baker, C.J., N.M. Mock, B.D. Whitaker, D.P. Roberts, C.P. Rice, K.L. Deahl, and A.A. Aver’yanov. 2005. Involvement of acetosyringone in plant–pathogen recognition. Biochem. Biophys. Res. Commun. 328: 130-136.
- Barbas, C., E.P. Moraes, and A. Villaseñor. 2011. Capillary electrophoresis as a metabolomics tool for non-targeted fingerprinting of biological samples. J. Pharm. Biomed. Anal. 55: 823-831.
- Beck, J.J., L. Smith, and N. Baig. 2014. An overview of plant volatile metabolomics, sample treatment and reporting considerations with emphasis on mechanical damage and biological control of weeds. Phytochem. Anal. 25: 331-341.
- Becker, L., V. Carré, A. Poutaraud, D. Merdinoglu, and P. Chaimbault. 2014. MALDI mass spectrometry imaging for the simultaneous location of resveratrol, pterostilbene and viniferins on grapevine leaves. Molecules 19: 10587-10600.
- Bednarek, P., B. Schneider, A. Svatoš, N.J. Oldham, and K. Hahlbrock. 2005. Structural complexity, differential response to infection and tissue specificity of indolic and phenylpropanoid secondary metabolism in Arabidopsis roots. Plant Physiol. 138: 1058-1070.
- Begley, P., S. Francis-McIntyre, W.B. Dunn, D.I. Broadhurst, A. Halsall, A. Tseng, J. Knowles, HUSERMET Consortium, R. Goodacre, and D.B. Kell. 2009. Development and performance of a gas chromatography-time-of-flight mass spectrometry analysis for large-scale nontargeted metabolomic studies of human serum. Anal Chem. 81: 7038-7046.
- Benkeblia, N., T. Shinano, and M. Osaki. 2007. Metabolite profiling and assessment of metabolome compartmen-tation of soybean leaves using non-aqueous fractionation and GC-MS analysis. Metabolomics 3: 297-305.
- Brechenmacher, L., Z. Lei, M. Libault, S. Findley, M. Sugawara, M.J. Sadowsky, L.W. Sumner, and G. Stacey. 2010. Soybean metabolites regulated in root hairs in response to the symbiotic bacterium Bradyrhizobium japonicum. Plant Physiol. 153: 1808-1822.
- Broeckling, C.D., D.V. Huhman, M.A. Farag, J.T. Smith, G.D. May, P. Mendes, R.A. Dixon, and L.W. Sumner. 2005. Metabolic profiling of Medicago truncatula cell cultures reveals the effects of biotic and abiotic elicitors on metabolism. J. Exp. Bot. 56: 323-336.
- Brown, M., W.B. Dunn, D.I. Ellis, R. Goodacre, J. Handl, J.D. Knowles, S. O’Hagan, I. Spasić, and D.B. Kell. 2005. A metabolome pipeline: from concept to data to knowledge. Metabolomics 1: 39-51.
- Burt, T., and S. Nandal. 2016. Pharmacometabolomics in early-phase clinical development. Clin. Transl. Sci. 9: 128-138.
- Cevallos-Cevallos, J.M., D.B. Futch, T. Shilts, S.Y. Folimonova, and J.I. Reyes-De-Corcuera. 2012. GC-MS metabolomic differentiation of selected citrus varieties with different sensitivity to citrus huanglongbing. Plant Physiol. Biochem. 53: 69-76.
- Chaurand, P., J.L. Norris, D.S. Cornett, J.A. Mobley, and R.M. Caprioli. 2006. New developments in profiling and imaging of proteins from tissue sections by MALDI mass spectrome-try. J. Proteome Res. 5: 2889-2900.
- Chen, Y.-C., E.C. Holmes, J. Rajniak, J.-G. Kima, S. Tang, C.R. Fischer, M.B. Mudgett, and E.S. Sattely. 2018. N-hydroxy-pipecolic acid is a mobile metabolite that induces systemic disease resistance in Arabidopsis. Proc. Natl. Acad. Sci. USA 115: E4920-E4929.
- Choi, Y.H., E.C. Tapias, H.K. Kim, A.W.M. Lefeber, C. Erkelens, J.Th.J. Verhoeven, J. Brzin, J. Zel, and R. Verpoorte. 2004. Metabolic discrimination of Catharanthus roseus leaves infected by phytoplasma using 1H-NMR spectroscopy and multivariate data analysis. Plant Physiol. 135: 2398-2410.
- Courant, F., J.-P. Antignac, G. Dervilly-Pinel, and B. Le Bizec. 2014. Basics of mass spectrometry based metabolomics. Proteomics 14: 2369-2388.
- Dai, H., C. Xiao, H. Liu, and H. Tang. 2010. Combined NMR and LC-MS analysis reveals the metabonomic changes in Salvia miltiorrhiza Bunge induced by water depletion. J. Proteome Res. 9: 1460-1475.
- Dauwe, R., K. Morreel, G. Goeminne, B. Gielen, A. Rohde, J. Van Beeumen, J. Ralph, A.-M. Boudet, J. Kopka, S.F. Rochange, C. Halpin, E. Messens, and W. Boerjan. 2007. Molecular phenotyping of lignin-modified tobacco reveals associated changes in cell-wall metabolism, primary metabolism, stress metabolism and photorespiration. Plant J. 52: 263-285.
- Dempsey, D.M.A., and D.F. Klessig. 2012. SOS - too many signals for systemic acquired resistance? Trends Plant Sci. 17: 538-545.
- Dixon, R.A., L. Achnine, P. Kota, C.-J. Liu, M.S.S. Reddy, and L. Wang. 2002. The phenylpropanoid pathway and plant defence-a genomics perspective. Mol. Plant Pathol. 3: 371-390.
- Dunn, W.B., D. Broadhurst, P. Begley, E. Zelena, S. Francis-McIntyre, N. Anderson, M. Brown, J.D. Knowles, A. Halsall, J.N. Haselden, A.W. Nicholls, I.D. Wilson, D.B. Kell, R. Goodacre, and HUSERMET Consortium. 2011. Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nat. Protoc. 6: 1060-1083.
- Ebel, J., A.R. Ayers, and P. Albersheim. 1976. Host-pathogen interactions: XII. Response of suspension-cultured soybean cells to the elicitor isolated from Phytophthora megasperma var. sojae, a fungal pathogen of soybeans. Plant Physiol. 57: 775-779.
- Fang, X., C.-Q. Yang, Y.-K. Wei, Q.-X. Ma, L. Yang, and X.-Y. Chen. 2011. Genomics grand for diversified plant secondary metabolites. Plant Divers. 33: 53-64.
- Fiehn, O. 2002. Metabolomics – the link between genotypes and phenotypes. Plant Mol. Biol. 48: 155-171. doi:10.1023/A:1013713905833
- Friesen, T.L., S.W. Meinhardt, and J.D. Faris. 2007. The Stagonospora nodorum-wheat pathosystem involves multiple proteinaceous host-selective toxins and corres-ponding host sensitivity genes that interact in an inverse gene-for-gene manner. Plant J. 51: 681-692.
- Giberti, S., C.M. Bertea, R. Narayana, M.E. Maffei, and G. Forlani. 2012. Two phenylalanine ammonia lyase isoforms are involved in the elicitor-induced response of rice to the fungal pathogen Magnaporthe oryzae. J. Plant Physiol. 169: 249-254.
- Goodacre, R., S. Vaidyanathan, W.B. Dunn, G.G. Harrigan, and D.B. Kell. 2004. Metabolomics by numbers: acquiring and understanding global metabolite data. Trends Biotechnol. 22: 245-252.
- Gottstein, D., and D. Gross. 1992. Phytoalexins of woody plants. Trees 6: 55-68.
- Gross, J.H. 2004. Mass spectrometry. Springer, Berlin, Heidelberg, Germany. 534 pp.
- Guo, Y., A. Graber, R.N. McBurney, and R. Balasubramanian. 2010. Sample size and statistical power considerations in high-dimensionality data settings: a comparative study of classification algorithms. BMC Bioinform. 11: 447.
- Haas, D., and G. Défago.2005. Biological control of soil-borne pathogens by fluorescent pseudomonads. Nat. Rev. Microbiol. 3: 307-319.
- Handelsman, J., and E.V. Stabb.1996. Biocontrol of soilborne plant pathogens. Plant Cell 8: 1855-1869.
- Hatada, K., and T. Kitayama. 2004. Introduction to NMR spectroscopy. Pages 1-42 in K. Hatada, and T. Kitayama (eds.), NMR spectroscopy of polymers. Springer, New York, USA.
- He, X.-Z., and R.A. Dixon. 2000. Genetic manipulation of isoflavone 7-O-methyltransferase enhances biosynthesis of 4'-O-methylated isoflavonoid phytoalexins and disease resistance in alfalfa. Plant Cell 12: 1689-1702.
- Hegeman, A.D. 2010. Plant metabolomics—meeting the analytical challenges of comprehensive metabolite analysis. Brief. Funct. Genom. 9: 139-148.
- Hong, Y.-S., A. Martinez, G. Liger-Belair, P. Jeandet, J.-M. Nuzillard, and C. Cilindre. 2012. Metabolomics reveals simultaneous influences of plant defence system and fungal growth in Botrytis cinerea infected Vitis vinifera cv. Chardonnay berries. J. Exp. Bot. 63: 5773-5785.
- Horgan, R.P., and L.C. Kenny. 2011. ‘Omic’ technologies: genomics, transcriptomics, proteomics and metabolomics. Obstet. Gynaecol. 13: 189-195.
- Huhman, D.V., and L.W. Sumner. 2002. Metabolic profiling of saponins in Medicago sativa and Medicago truncatula using HPLC coupled to an electrospray ion-trap mass spectrometer. Phytochemistry 59: 347-360. doi:10.1016/S0031-9422(01)00432-0
- Khakimov, B., S. Bak, and S.B. Engelsen. 2014. High-throughput cereal metabolomics: current analytical technologies, challenges and perspectives. J. Cereal Sci. 59: 393-418.
- Krishnan, P., N.J. Kruger, and R.G. Ratcliffe. 2005. Metabolite fingerprinting and profiling in plants using NMR. J. Exp. Bot. 56: 255-265.
- Lattanzio, V., V.M.T. Lattanzio, and A. Cardinali. 2006. Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects. Pages 23-67 in F. Imperato (ed.), Phytochemistry: advances in research. Research Signpost, Trivandrum, Kerala, India.
- Lima, M.R.M., M.L. Felgueiras, G. Graça, J.E.A. Rodrigues, A. Barros, A.M. Gil, and A.C.P. Dias. 2010. NMR metabolomics of esca disease-affected Vitis vinifera cv. Alvarinho leaves. J Exp. Bot. 61: 4033-4042.
- López-Gresa. M.P., F. Maltese, J.M. Bellés, V. Conejero, H.K. Kim, Y.H. Choi, and R. Verpoorte. 2010. Metabolic response of tomato leaves upon different plant-pathogen interac-tions. Phytochem. Anal. 21: 89-94.
- Luan, J.-B., D.-M. Yao, T. Zhang, L.L. Walling, M. Yang, Y.-J. Wang, and S.-S. Liu. 2013. Suppression of terpenoid synthesis in plants by a virus promotes its mutualism with vectors. Ecol. Lett. 16: 390-398.
- Lui, L.H., A. Vikram, Y. Abu-Nada, A.C. Kushalappa, G.S.V. Raghavan, and K. Al-Mughrabi. 2005. Volatile metabolic profiling for discrimination of potato tubers inoculated with dry and soft rot pathogens. Am. J. Potato Res. 82: 1-8.
- Mauck, K.E., C.M. De Moraes, and M.C. Mescher. 2014. Biochemical and physiological mechanisms underlying effects of Cucumber mosaic virus on host-plant traits that mediate transmission by aphid vectors. Plant Cell Environ. 37: 1427-1439.
- Miyata, K., M. Miyashita, R. Nose, Y. Otake, and H. Miyagawa. 2006. Development of a colorimetric assay for determining the amount of H2O2 generated in tobacco cells in response to elicitors and its application to study of the structure-activity relationship of flagellin-derived peptides. Biosci. Biotechnol. Biochem. 70: 2138-2144.
- Mucha, P., J. Ruczynski, M. Dobkowski,E. Backtrog, and P. Rekowski. 2019. Capillary electrophoresis study of systemin peptides spreading in tomato plant. Electrophoresis 40: 336-342.
- Mutuku, J.M., and A. Nose. 2012. Changes in the contents of metabolites and enzyme activities in rice plants responding to Rhizoctonia solani Kuhn infection: activation of glycolysis and connection to phenylpropanoid pathway. Plant Cell Physiol. 53: 1017-1032.
- Návarová, H., F. Bernsdorff, A.-C. Döring, and J. Zeier. 2012. Pipecolic acid, an endogenous mediator of defense amplification and priming, is a critical regulator of inducible plant immunity. Plant Cell 24: 5123-5141.
- Niederbacher, B., J.B. Winkler, and J.P. Schnitzler. 2015. Volatile organic compounds as non-invasive markers for plant phenotyping. J. Exp. Bot. 66: 5403-5416.
- Norris, J.L., and R.M. Caprioli. 2013. Analysis of tissue specimens by matrix-assisted laser desorption/ionization imaging mass spectrometry in biological and clinical research. Chem. Rev. 113: 2309-2342.
- Okazaki, Y., and K. Saito. 2012. Recent advances of metabolomics in plant biotechnology. Plant Biotechnol. Rep. 6: 1-15.
- Oksman-Caldentey, K.-M., and D. Inzé. 2004. Plant cell factories in the post-genomics era: new ways to produce designer secondary metabolites. Trends Plant Sci. 9: 433-440.
- Olson, B.H., and G.L. Goerner. 1965. Alpha sarcin, a new antitumor agent. I. Isolation, purification, chemical composition, and the identity of a new amino acid. Appl. Microbiol. 13: 314-321.
- Ongena, M., E. Jourdan, A. Adam, M. Paquot, A. Brans, B. Joris, J.-L. Arpigny, and P. Thonart. 2007. Surfactin and fengycin lipopeptides of Bacillus subtilis as elicitors of induced systemic resistance in plants. Environ. Microbiol. 9: 1084-1090.
- Pearce, G., D. Strydom, S. Johnson, and C.A. Ryan. 1991. A polypeptide from tomato leaves induces wound-inducible proteinase inhibitor proteins. Science 253: 895-897.
- Perrin, D.R., and W. Bottomley. 1961. Pisatin: an antifungal substance from Pisumsativum L. Nature 191: 76-77.
- Piasecka, A., N. Jedrzejczak-Rey, and P. Bednarek. 2015. Secondary metabolites in plant innate immunity: conserved function of divergent chemicals. New Phytol. 206: 948-964.
- Pushpa, D., K.N. Yogendra, R. Gunnaiah, A.C. Kushalappa, and A. Murphy. 2014. Identification of late blight resistance-related metabolites and genes in potato through non-targeted metabolomics. Plant Mol. Biol. Report. 32: 584-595.
- Rao, Q., W. Guo, and X. Chen. 2015. Identification and characterization of an antifungal protein, AfAFPR9, produced by marine-derived Aspergillus fumigatus R9. J. Microbiol. Biotechnol. 25: 620-628.
- Roessner, U., A. Luedemann, D. Brust, O. Fiehn, T. Linke, L. Willmitzer, and A.R. Fernie. 2001. Metabolic profiling allows comprehensive phenotyping of genetically or environ-mentally modified plant systems. Plant Cell 13: 11-29.
- Röhlig, R.M., J. Eder, and K.-H. Engel. 2009. Metabolite profiling of maize grain: differentiation due to genetics and environment. Metabolomics 5: 459.
- Ryan, C.A. 2000. The systemin signaling pathway: differential activation of plant defensive genes. Biochim. Biophys. Acta 1477: 112-121. doi:10.1016/S0167-4838(99)00269-1
- Sato, S., T. Soga, T. Nishioka, and M. Tomita. 2004. Simultaneous determination of the main metabolites in rice leaves using capillary electrophoresis mass spectro-metry and capillary electrophoresis diode array detection. Plant J. 40: 151-163.
- Shah, J., R. Chaturvedi, Z. Chowdhury, B. Venables, and R.A. Petros. 2014. Signaling by small metabolites in systemic acquired resistance. Plant J. 79: 645-658.
- Shah, J., and J. Zeier. 2013. Long-distance communication and signal amplification in systemic acquired resistance. Front. Plant Sci. 4: 30.
- Shen, Y., J. Li, J. Xiang, J. Wang, K. Yin, and Q. Liu. 2019. Isolation and identification of a novel protein elicitor from a Bacillus subtilis strain BU412. AMB Expr 9: 117.
- Siricord, C., and P.A. O’Brien. 2008. MALDI-TOF mass spectrometry can be used for detection of pathogenic microorganisms in soil. Australas. Plant Pathol. 37: 543-545.
- Skirycz, A., S. De Bodt, T. Obata, I. De Clercq, H. Claeys, R. De Rycke, M. Andriankaja, O. Van Aken, F. Van Breusegem, A.R. Fernie, and D. Inze. 2010. Developmental stage specificity and the role of mitochondrial metabolism in the response of Arabidopsis leaves to prolonged mild osmotic stress. Plant Physiol. 152: 226-244.
- Soares, M.S., D.F. da Silva, M.R. Forim, M.F.G.F. da Silva, J.B. Fernandes, P.C. Vieira, D.B. Silva, N.P. Lopes, S.A. de Carvalho, A.A. de Souza, and M.A. Machado. 2015. Quantification and localization of hesperidin and rutin in Citrus sinensis grafted on C. limonia after Xylella fastidiosa infection by HPLC-UV and MALDI imaging mass spectrometry. Phytochemistry 115: 161-170.
- Soylu, S. 2006. Accumulation of cell-wall bound phenolic compounds and phytoalexin in Arabidopsis thaliana leaves following inoculation with pathovars of Pseudomonas syringae. Plant Sci. 170: 942-952.
- Stitt, M., and A.R. Fernie. 2003. From measurements of metabolites to metabolomics: an ‘on the fly’ perspective illustrated by recent studies of carbon-nitrogen interac-tions. Curr. Opin. Biotechnol. 14: 136-144. doi:10.1016/S0958-1669(03)00023-5
- Suharti, W.S., A. Nose, and S.-H. Zheng.2016. Metabolite profiling of sheath blight disease resistance in rice: in the case of positive ion mode analysis by CE/TOF-MS. Plant Prod. Sci. 19: 279-290.
- Sumner, L.W., P. Mendes, and R.A. Dixon. 2003. Plant metabolomics: large-scale phytochemistry in the functional genomics era. Phytochemistry 62: 817-836. doi:10.1016/S0031-9422(02)00708-2
- Tolstikov, V.V., and O. Fiehn. 2002. Analysis of highly polar compounds of plant origin: combination of hydrophilic interaction chromatography and electrospray ion trap mass spectrometry. Anal. Biochem. 301: 298-307.
- Trygg, J., E. Holmes, and T. Lundstedt. 2007. Chemometrics in metabonomics. J. Proteome Res. 6: 469-479.
- Tugizimana, F., A.T. Djami-Tchatchou, P.A. Steenkamp, L.A. Piater, and I. Dubery. 2019. Metabolomic analysis of defense-related reprogramming in Sorghum bicolor in response to Colletotrichum sublineolum infection reveals a functional metabolic web of phenylpropanoid and flavonoid pathways. Front. Plant Sci. 9: 1840.
- Tugizimana, F., L. Piater, and I. Dubery. 2013. Plant metabolomics: a new frontier in phytochemical analysis. S. Afr. J. Sci. 109.
- Tzin, V., and G. Galili. 2010. The biosynthetic pathways for shikimate and aromatic amino acids in Arabidopsis thaliana. Arabidopsis Book 8: e0132.
- Urbanczyk-Wochniak, E., A. Luedemann, J. Kopka, J. Selbig, U. Roessner-Tunali, L. Willmitzer, and A.R. Fernie. 2003. Parallel analysis of transcript and metabolic profiles: a new approach in systems biology. EMBO Rep. 4: 989-993.
- Van Loon, L.C., and P.A.H.M. Bakker. 2003. Signalling in rhizobacteria-plant interactions. Pages 297-330 in H. de Kroon, and E.J.W. Visser (eds.), Root ecology. Springer, Berlin, Heidelberg, Germany.
- Van Loon, L.C., and B.R. Glick. 2004. Increased plant fitness by rhizobacteria. Pages 177-205 in H. Sandermann (ed.), Molecular ecotoxicology of plants. Springer, Berlin, Heidelberg, Germany.
- Wagner, C., M. Sefkow, and J. Kopka. 2003. Construction and application of a mass spectral and retention time index database generated from plant GC/EI-TOF-MS metabolite profiles. Phytochemistry 62: 887-900. doi:10.1016/S0031-9422(02)00703-3
- Wang, B., X. Yang, H. Zeng, H. Liu, T. Zhou, B. Tan, J. Yuan, L. Guo, and D. Qiu. 2012. The purification and characterization of a novel hypersensitive-like response-inducing elicitor from Verticillium dahliae that induces resistance responses in tobacco. Appl. Microbiol. Biotechnol. 93: 191-201.
- Wang, J.-Y., Y. Cai, J.-Y. Gou, Y.-B. Mao, Y.-H. Xu, W.-H. Jiang, and X.-Y. Chen. 2004. VdNEP, an elicitor from Verticillium dahliae, induces cotton plant wilting. Appl. Environ. Microbiol. 70: 4989-4995.
- Wang, N., M. Liu, L. Guo, X. Yang, and D. Qiu. 2016. A novel protein elicitor (PeBA1) from Bacillus amyloliquefaciens NC6 induces systemic resistance in tobacco. Int. J. Biol. Sci. 12: 757-767.
- Ward, J.L., J.M. Baker, A.M. Llewellyn, N.D. Hawkins, and M.H. Beale. 2011. Metabolomic analysis of Arabidopsis reveals hemiterpenoid glycosides as products of a nitrate ion-regulated, carbon flux overflow. Proc. Natl. Acad. Sci. USA 108: 10762-10767.
- Wnendt, S., N. Ulbrich, and U. Stahl. 1994. Molecular cloning, sequence analysis and expression of the gene encoding an antifungal-protein from Aspergillus giganteus. Curr. Genet. 25: 519-523.
- Yang, W.-L., and M.A. Bernards.2007. Metabolite profiling of potato (Solanum tuberosum L.) tubers during wound-induced suberization. Metabolomics 3: 147-159.
- Yogendra, K.N., A.C. Kushalappa, F. Sarmiento, E. Rodriguez, and T. Mosquera. 2014. Metabolomics deciphers quanti-tative resistance mechanisms in diploid potato clones against late blight. Funct. Plant Biol. 42: 284-298.
- Zhang, A., H. Sun, P. Wang, Y. Han, and X. Wang. 2012. Modern analytical techniques in metabolomics analysis. Analyst 137: 293-300.
Liste des tableaux
Table 1
List of some metabolomic techniques and their use in studying plant pathogen interaction