Résumés
Abstract
The objectives of this study were to determine the qualitative production of extracellular enzymes produced by ten endophytic fungi and to investigate their antifungal potential against some phytopathogenic fungi namely, Alternaria alternata, Botrytis cinerea, Fusarium oxysporum and Rhizoctonia solani. In addition, the endophytic fungal isolates were screened for enzyme production by plate assay method. All endophytic fungi were able to produce proteases and cellulases with different levels except Alternaria alternata. Meanwhile, Fusarium, Alternaria, Nigrospora and Phoma species produced amylase. None of the tested endophytic fungi showed laccase production. Endophytic fungi filtrates revealed variable antifungal activities against the tested phytopathogenic fungi with Curvularia lunata filtrate being the most effective. This filtrate induced 48% and 80% growth inhibition of B. cinerea and R. solani, respectively. The phytochemical analysis of the endophytic fungi crude extract disclosed the presence of alkaloids and terpenoids. Morphological observations by optical microscope showed uncommon hyphal deformation and enlargement of cytoplasmic vacuoles of R. solani when treated with C. lunata filtrate. Furthermore, C. lunata (hyphal suspension and filtrate) was examined to control root rot caused by R. solani on faba bean plants in vivo. Both antagonistic treatments significantly reduced root rot severity.
Keywords:
- biological control,
- Curvularia lunata,
- endophytes,
- extracellular enzymes,
- pathogenic fungi,
- secondary metabolites
Résumé
Les objectifs de cette étude étaient de déterminer la production qualitative des enzymes extracellulaires de dix champignons endophytes et d’étudier le potentiel antimicrobien de leurs filtrats sur certains champignons phyto-pathogènes. Les dosages enzymatiques ont démontré qu’il y avait une variation dans la production de différentes enzymes extracellulaires parmi les champignons endophytes testés. Tous les champignons endophytes étaient capables de produire des protéases et de la cellulase avec des niveaux différents sauf Alternaria alternata. Pourtant, les espèces Fusarium, Alternaria, Nigrospora et Phoma étaient capables de produire l’amylase. Aucun des champignons endophytes testés n’a produit de laccase. Les filtrats de champignons endophytes ont révélé des activités antifongiques variables contre les champignons phytopathogènes testés, dont le plus efficace était le filtrat de Curvularia lunata. Ce filtrat a induit une inhibition de croissance de 48 % et 80 % de Botrytis cinerea et Rhizoctonia solani respectivement. L’analyse phytochimique de l’extrait brut de champignons endophytes a révélé la présence d’alcaloïdes et de terpénoïdes. Les observations morphologiques au microscope optique ont montré une déformation hypale et un élargissement des vacuoles cytoplasmiques de R. solani lorsqu’il est traité avec du filtrat de C. lunata. De plus, C. lunata (suspension d’hypales et filtrat) a été examiné pour lutter contre la pourriture des racines causée par R. solani sur des plants de fèves in vivo. Les deux traitements antagonistes ont considérablement réduit la gravité de la pourriture des racines.
Mots-clés :
- lutte biologique,
- Curvularia lunata,
- endophytes,
- enzymes extracellulaires,
- champignons pathogènes,
- métabolites secondaires
Corps de l’article
INTRODUCTION
Plant pathogens are harmful worldwide as they can cause pre- and post-harvest losses of different crops, and thus, threatening crop security, especially in developing countries. Around $220 billion are globally lost every year due to the attack of economic crops by different pathogens (Food and Agriculture Organization of the United Nations, 2019). Management procedures of plant diseases usually demand the use of one, or a combination of resistant cultivars, cultural and chemical control measures. The overuse of synthetic chemicals poses a potential risk to humans and harmful effects to the environment by reducing agricultural sustainability. In addition, the excessive use of these chemicals increases the development of pathogen resistance, leading to failures in disease control (Alori and Babalola 2018). As a result, a strenuous search for new, potent antimicrobial agents is necessary, which is facilitated by exploring new niches and habitats. The majority of biocontrol agents are obtained through isolation by screening organisms from rhizosphere or endophyte populations to exert an in vitro inhibitory effect on the target pathogen. Furthermore, there are other characteristics needed for a reliable and successful biocontrol agent as its ability for mass production and persistence under field conditions.
Endophytic fungi represent a wide diversity of microbial adaptations that have evolved in special and unusual environments, making them a great source of study and research for agricultural, industrial, and medical usage (White et al. 2019).
Endophytes are symbiotic micro-organisms that co-exist in close relationship with plant tissues without causing any visible pathological symptoms on the host. They colonize the plant interior and contribute to the growth development, fitness, and cause higher stress resistance (Khare et al. 2018; Kusari et al. 2014). Several endophytic fungal genera are often involved in complex relationships between the synthesis, degradation and accumulation of secondary metabolites that may have prospective biological action against pests or pathogens (Anyasi and Atagana 2019; Kusari et al. 2014). Bioactive secondary metabolites such as alkaloids, terpenoids, steroids, quinones, isocoumarins, lignans, phenylpropanoids, phenols, and lactones, which are known to have antimicrobial activity, are commonly produced by endophytes (Saikkonen et al. 2004). In addition, endophytes initiate actions to stimulate plant growth through the production of essential biochemical resources such as phytohormones and extra-cellular enzymes which help host plants to resist biotic and abiotic stresses (Chathurdevi and Gowrie 2016). Moreover, endophytes can induce the expression of many host defence-related genes (Busby et al. 2016; Mejía et al. 2014). On the other hand, the host plant provides a protective sanctuary to endophytes reproduction and nutrients to grow inside plant tissues without compromising its own growth resources (Khan et al. 2015). Generally, the biocontrol effect is dependent on the endophyte isolate, pathogen, plant species, and environmental conditions. In addition to helping the host to resist any pathogenic invasion and acquiring the essential nutrition from the host, these extracellular enzymes display variably important applications in industry, like the production of biofuel, detergents, food and pharmaceutical products (Firáková et al. 2007).
The current study aimed to evaluate the ability of ten endophytic fungi isolated from two medicinal plants and two weeds to produce enzymes in vitro and to investigate the antagonistic activity of these endophytes against some widespread pathogenic fungi of many economically important crops. Additionally, the investigation aimed to study the effectiveness of the secondary metabolites of the isolate exhibited potential as antimicrobial resources for plant protection to R. solani in a pot experiment as an innovative solution to the faba bean root rot problem.
MATERIALS AND METHODS
Microorganisms
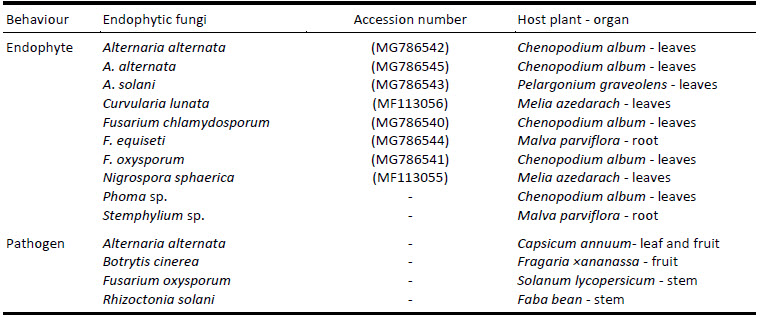
Strains of the endophytic fungi (Table 1), used in the current assay, have been isolated in previous study and were morphologically and molecularly characterized (Saad et al. 2019). In previous experiments, all endophytes proved to be true endophyte. The pathogenic fungi (Table 1) came from a collection at the University of Alexandria, Plant Pathology Department. Endophytic and pathogenic fungi cultures were preserved on Potato Dextrose Agar (PDA) slant agar at 4 °C for further experiments.
Plate based assay for extracellular enzymes
The production of enzymes, amylase, cellulose, esterase, protease and laccase, by the tested fungal endophytes, was qualitatively determined by using Hankin and Anagnostakis method (1975). Substrate agar plates, incubated without fungi, were used as a negative control. The isolates were tested for cellulase production in Glucose Yeast Peptone (GYP) agar (glucose 10.0 g; yeast extract 0.1 g; peptone 0.5 g; soluble starch 2 g; agar 16.0 g L-1; final pH = 6.0) amended with 0.5% Na-carboxy-methylcellulose as a carbon source in suspension. After incubation, 0.2% aqueous Congo Red and 1M NaCl were used to allow the visualization of clear zone surrounding the colony indicating the cellulase activity. Meanwhile, amylase activity was assessed by growing the fungi on GYP. After incubation, the plates were flooded with 1% iodine in 2% potassium iodide. A clear zone surrounding the colony was considered positive for amylase enzyme. For esterase activity, the fungi were grown on peptone agar medium (peptone 10.0 g; NaCl 5.0 g; CaCl2·2H2O 0.1 g; agar 16.0 g; distilled water 1 L; pH = 6.0) supplemented with 1% Tween 80 sterilized separately. A clear zone around the colony indicated esterase positive fungi. Protease activity was assayed by growing the fungi on GYP agar medium which contains 0.4% gelatin, pH 6. After the incubation period, plates were covered with saturated aqueous ammonium sulfate. The undigested gelatin will precipitate with ammonium sulfate and digested area around the fungal colony would appear as clear zone. For laccase activity, fungi were grown on GYP agar amended with 0.05 g L-1 of 1-Naphthol. On oxidation of 1-Naphthol by laccase, the medium changed from clear to blue.
Screening of the endophytic isolates against pathogenic fungi
In order to screen the endophytic fungi for its antifugual activities, the ten endophytic isolates were grown in 100 mL of Potato Dextrose Broth (PDB) in 250 mL Erlenmeyer flask for three weeks at 25 ± 2 °C in dark condition. Cell free supernatant was prepared by filtration twice with Whatman® qualitative filter paper, Grade 1 (Whatman International Ltd, Maidstone, UK) followed by filtration through a 0.45 mm Millipore membrane to recover sterilized culture filtrate. The filtrates were preserved in refrigerators at 4 °C. The antifungal activity of crude filtrates of the endophytic fungi species was checked using the agar well diffusion method (Deans and Ritchie 1987). The test was performed against the mycelial growth of the pathogenic fungi R. solani, A. alternata, F. oxysporum and B. cinerea. A 500 μL sample of culture filtrate was added to the wells (8 mm diameter) of PDA plates then, the plates were left for 2 h at room temperature to allow the diffusion of filtrates into the agar before inoculation with test micro-organisms. A plug (6 mm diameter) of seven-day-old mycelia of the pathogenic fungi was placed opposite to the well and incubated at 25 ± 2 °C. Plates without the endophytic fungi filtrates served as control using sterilized PDB. When the mycelium growth reached the edges of the control plate, the percentage of the linear mycelial growth inhibition (PGI) was calculated according to the formula: PGI (%) = (A-B/A) x 100, where A is the fungal radial growth (mm) of the control plates and B is the fungal radial growth (mm) in the treated plates. Each experiment was run in triplicate and was repeated at least three times.
Table 1
Characteristics of the endophytes and the pathogenic fungi
Microscopic examination
Light microscopy (Olympus Soft Imaging Solutions GmbH) was utilized to check the effect of the filtrate of the potent endophytic against R. solani. Mycelial samples from the confrontation region between the filtrate and R. solani, as well as samples from single cultures of the fungus (control treatment) on PDA were fixed in 2% (v/v) glutaraldehyde in 0.1 M cacodylate buffer (pH 7.4) with 1 mM CaCl and 1% (w/v) sucrose for 3 h at room temperature. Samples were then rinsed six times with the same buffer and postfixed with 1% (w/v) osmium tetroxide for 2 h at room temperature. After thoroughly rinsing with 0.1 M cacodylate buffer (pH 7.4), samples were dehydrated in a graded ethanol series. Fully dehydrated samples were moved from absolute ethanol through a 2:2 mixture of ethanol and propylene oxide to pure propylene oxide. Samples were infiltrated through a series of Epon-Araldite mixture resin in propylene oxide, then embedded with with fresh 100% resin in moulds and polymerized at 65 °C for 36 h. Ultrathin sections cut with a glass knife were collected and examined.
Screening of phytochemicals
The crude extract of the most active endophyte, C. lunata was subjected to chemical analysis to detect the presence of some secondary metabolites following standard procedures according to Bhardwaj et al. (2015) as follows:
Alkaloids: 1 mL of endophytic crude extract was dissolved in 2N HCl solution then was treated with few drops of Mayer’s reagent. The presence of alkaloids was indicated by the formation of cream-coloured precipitate.
Flavonoids: A few drops of 20% NaOH solution was added to 1 mL of endophytic crude extract. Observation of yellow colour on addition of acid altered to colourless solution presented the presence of flavonoids.
Phenols: The endophytic extract was dissolved in 5 mL of distilled water and few drops of neutral 5% ferric chloride solution were added. A dark green colour pointed out the presence of phenolic compounds.
Saponins: Distilled water was added to the endophytic extract, vigorously shaken, then it was allowed to stand for 10 min. The presence of saponins was determined by formation of a fairly stable emulsion.
Steroids: 1 mL of chloroform was added to the crude endophytic extract then the mixture was treated with acetic anhydride and few drops of concentrated H2SO4. The formation of blue-green ring indicated the presence of steroids.
Tannins: The endophytic crude extract was treated with alcoholic FeCl3 solution. A bluish-black colour was formed which disappeared when few drops of dilute H2SO4 were added then yellowish-brown precipitate was formed, which indicated the existence of tannins.
Terpenoids: 2 mL of chloroform was mixed with 1 mL of endophytic crude extract then 3 mL of concentrated H2SO4 was added to form a layer. At the interface layer, a reddish-brown precipitate colouring was formed as an indication of the presence of terpenoids.
Evaluation of the impact of C. lunata on disease incidence and severity
Based on the in vitro experiment results, C. lunata was evaluated under greenhouse conditions against R. solani root rot disease in faba bean (cv. Giza2). In order to know if the presence of the endophytic filtrate was able to either prevent R. solani infections or reduce its growth, two seed treatments were performed using culture filtrate and hyphal spore suspension. To perform the soil infestation with R. solani, suspension of mycelia of the pathogen was mixed with the upper layer of the soil (15 mL per pot). After the soaking treatments, seeds were planted in a pot (20 cm x 17 cm) containing autoclaved soil (1 peat: 1 peat moss). The pots were divided into six groups and treated as follows: a) faba bean seeds of the first group were sown in sterilized soil free from pathogens to serve as a control (healthy plants); b) seeds of the second group were sown in sterilized soil free from pathogens after being soaked for one hour in C. lunata filtrate; c) seeds of the third group were sown in sterilized soil free from pathogens after being soaked for one hour in C. lunata hyphal-spore suspension; d) seeds of the fourth group were sown in sterilized soil infested with R. solani (infected plants); e) seeds of the fifth group were sown in sterilized soil infested with R. solani after being soaked for one hour in C. lunata filtrate; and f) seeds of the sixth group were sown in sterilized soil infested with R. solani after being soaked for one hour in C. lunata hyphal-spore suspension. All pots were kept under greenhouse conditions with 20 °C day and 16 °C night temperature and watered when necessary, throughout the experimental period. Four replicate plants were inoculated in a completely rando-mized design, and the experiment was repeated twice.
When the plants were 45 days old, they were carefully uprooted from the soil of each treatment to assess dry weights of shoots and roots. Root disease severity was assessed at each destructive sampling point on soil-free plants on scales from 0 to 5; 0 = no lesion, clean roots; 1 = small lesion on tap root; 2 = necrosis of up to 30%; 3 = necrosis covering 31–60% of the tap root; 4 = necrosis covering 61–99% of the tap root; 5 = completely severed tap root (Khangura et al. 1999). The disease incidence was calculated as the number of rotted plants in each pot relative to the total number of examined plants. The disease index (expressed as a percentage) was determined using the formula: Disease index = [Σ scale (No. infected plants x severity category) / (total examined/tested plants × upper severity category)] × 100.
Statistical analyses
Statistical analyses were carried out according to general linear model procedure of SAS (version 9.4; SAS institute Inc., Cary, NC). Data were subjected to one-way analysis of variance (ANOVA), and the means were compared by using LSD procedure (P < 0.05) and were disposed as mean ± standard deviation (SD). Percentage data were arcsine transformed before being subjected to ANOVA to normalize the data and stabilize variances throughout the data range. Line figures were drawn using Microsoft Excel 2016.
RESULTS
Production of extracellular enzymes
Ten endophytic fungi were screened to examine their ability to produce some specific enzymes (amylase, cellulase, lipase, laccase and protease) on GYP medium supplemented by appropriate carbon source. The formation of clearing or colour reaction zone surrounding the mycelium was considered as positive result. The qualitative results of endophytic fungal enzyme production are presented in Table 2. Enzyme assays demonstrated that there was a variation in the production of different extracellular enzymes by the tested endophytic fungi. Protease activity was detected in all tested fungi in a weak level except with A. alternata (MG786545), the activity was moderate. On the other hand, none of the tested endophytes exhibited laccase activity. Two species, A. solani (MG786543) and F. chlamydosporum (MG786540) were able to degrade other tested substrates. Regarding amylase production, only 60% of tested species were positive, whereas, F. oxysporum (MG786541) was the highest producer of the enzyme. F. oxysporum (MG786541) with A. solani (MG786543) were recorded as high producers of cellulase among the 90% positive fungi. Esterase production was poorly detected, where only 40% were positive with C. lunata (MF113056), the highest in enzyme production.
Table 2
Endophytic fungi isolated from plant leaves and root and their ability to produce enzymes on solid media
(+++) = high reaction, halo > 5 mm; (++) = moderate activity, halo < 5 mm; (+) = slight activity, halo < 3 mm; (-) = no enzymatic activity.
Screening of the endophytic isolates against pathogenic fungi
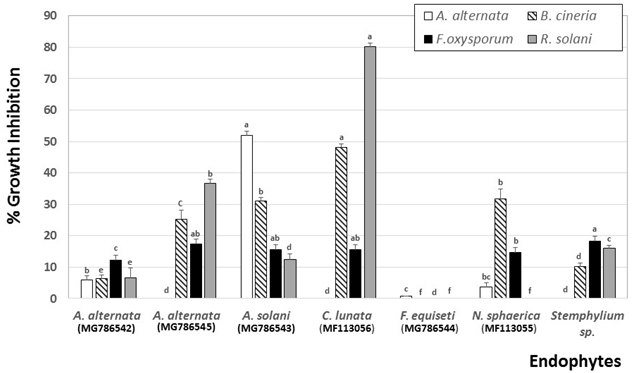
The in vitro effect of the crude cell-free supernatants of the tested endophytic fungal species against the linear mycelial growth of the pathogenic fungi A. alternata, B. cinerea, F. oxysporum and R. solani is presented in Fig. 1. The results showed that some crude extracts of endophytic fungi exhibited antifungal activity, inhibiting the mycelial growth of different tested pathogens. C. lunata (MF113056) was effective as potential biological control agent but its efficiency varied from strain to strain. The strongest antifungal activity against the tested strains R. solani and B. cinerea was exhibited by C. lunata (MF113056) with a percentage of inhibition that reached 80% and 48%, respectively. The antifungal activities of the other tested endophytic supernatants against B. cinerea led to less than 30% growth inhibition. Despite being effective against the four tested fungal strains, the fungicidal activity of A. solani (MG786543) did not reach more than 52% inhibition for A. alternata. Six supernatants exhibited antifungal activity toward F. oxysporum with a percentage of inhibition between 13% and 19%. The crude fungal supernatants of F. chlamydosporum, F. oxysporum and Phoma sp. did not exihibit growth suppressing effects on the four tested plant pathogenes. Results of the well diffusion experiments demonstrated a strong antagonistic potential of C. lunata filtrate against R. solani (Fig. 1). From the previous data, it is obvious that R. solani was more sensitive to secondary metabolites produced by C. lunata (MF113056). On the basis of the antifungal potential among the ten tested endophytes, C. lunata (MF113056) was considered for further studies.
Figure 1
Fungal growth inhibition (%) of A. alternata, B. cinerea, F. oxysporum and R. solani by endophytic fungal filtrates using well diffusion technique
Light microscope studies of R. solani hyphae
The clear zone between C. lunata filtrate and R. solani was examined using light microscope (Fig. 2). The mycelia of R. solani in the control treatment were in good condition, smooth and full, whereas, the mycelia treated with C. lunata filtrate accumulated melanin (brown coloration) at the edge of the growth of the confrontation area. The light microscopy study revealed prominent deformation and developed swelling of treated R. solani hyphae with C. lunata filtrate. In addition, the cell walls of R. solani became thinner and malformed with massive distortion of fungal morphologies such as leaked out protoplasm, and cracked mycelia. Treated R. solani also displayed enlargement of cytoplasmic vacuoles, whereas the untreated mycelia exhibited direct and balanced normal growth, branching and presented massive surface prominence. The cell wall was intact and thick and the cytoplasm was uniform.
Phytochemical analysis C. lunata filtrate
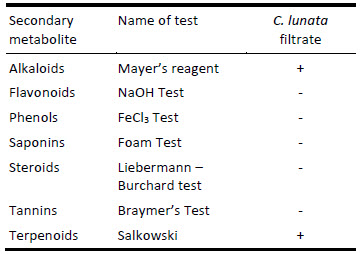
Phytochemical screening using qualitative analysis was carried out to assess the diversity of chemical compounds produced by C. lunata (MF113056). The phytochemical analysis of the endophytic fungi crude extract revealed the presence of alkaloids and terpenoids as illustrated in Table 3.
Figure 2
Morphology of R. solani under light microscope. (a) Untreated control group. (b) Treated group with C. lunata culture filtrate.
Table 3
Preliminary qualitative screening of secondary metabolites of C. lunata (MF113056) filtrate
Effect of C. lunata on the severity of Rhizoctonia root rot infecting faba bean plants
Data on the effect of the various treatments on disease severity and treatment efficacy on faba bean plants inoculated with R. solani at seeding time are reported in Table 4 and Fig. 3. Faba bean plant’s growth (cv. Giza2) was markedly inhibited due to the infection with R. solani as compared to the healthy plants. Infected plants were severely stunted and visible lesions were developed on roots as soon as 10 days after planting. Symptoms progressed to cover around 80% of the tap root (disease score 4) by the end of the experiment (Fig. 3d). The major symptoms of the disease included root rot and canker on the hypocotyl. Foliage was yellow, wilted, and eventually turned brown and dry. The tap roots were brown and necrotic; plants eventually died. The results showed that pathogen infection decreased dry weight of shoots and roots of the cultivar as compared with controls. No symptoms of root disease were observed in the non-inoculated treatments (Fig. 3a).
Table 4
Influence of filtrate and spore suspension of C. lunata on the incidence and severity of faba bean root rot disease under greenhouse condition
* Different letters indicate significant differences among treatments within the same column according to least significant difference test (P ≤ 0.05).
Figure 3
Suppression of Rhizoctonia root rot disease severity by endophytic fungi on faba bean: (a) Control (−) uninoculated plants. (b) Treated plants with C. lunata suspension. (c) Treated plants with C. lunata filtrates. (d) Control (+) inoculated plants with R. solani only. (e) Inoculated plants with R. solani and C. lunata suspension. (f) Inoculated plants with R. solani and C. lunata filtrates. Bar = 5cm.
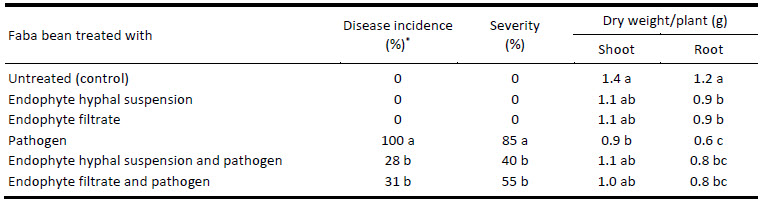
Data presented in Table 4 revealed that the maximum disease incidence (100%) and severity (85%), obviously, accompanied the treatment with the pathogen alone. Meanwhile, treating the faba bean with the endophyte hyphal suspension or filtrate, in presence of the pathogen, significantly reduced its negative impact on the plant, by reducing the disease incidence to 28% and 31% and the disease severity to 40% and 55%, in case of hyphal suspension, and filtrate, respectively.
This was reflected on the faba bean dry shoot and root weights. Application of both formulation of C. lunata caused a significant increase in growth parameters of both infected and non-infected plants as compared with untreated healthy plants (control). Highest significant shoot (1.4 g) and root (1.2 g) weights were characteristics of the untreated faba bean plants (control), while in presence of the pathogen alone, the shoot and root dry weights were reduced by 36% and 50%, respectively. The addition of endophyte hyphal suspension or filtrate, in presence of the pathogen, suppressed the negative effect of the pathogen alone, and lifted up the shoot and root dry weights of the plant. These effects were significantly similar to the addition of endophyte hyphal suspension or filtrate alone without pathogen.
Data presented in Table 4 showed that soil infected with R. solani (control) scored the lowest dry weight of root and shoot of faba bean compared to all other treatments after 45 days from planting. Significant difference was recorded between negative and positive controls. Despite the non-significant difference between treatment with filtrate and with hyphal suspension of C. lunata (MF113056), both treatments significantly improved shoot and root dry weight of faba bean plants in the infected plants with R. solani.
DISCUSSION
Endophytic microorganisms are associated with living tissues, and may, in some way, contribute to the well-being of the plant. They are not destructive, do not cause diseases to plants, and are different from epiphytic microbes which live on the surface of plant organs and tissues (Hallmann et al. 1997). One of the most important assets of endophytes, especially fungi, is associated with their bioactive potential.
An assortment of enzymes is known to be generated by endophytes such as amylase, cellulase, and pectinase (Bezerra et al. 2015; Khan et al. 2017). This study verified that production of cellulase was common among 90% of isolates, followed by amylase (60%). Fungal enzymes, particularly cellulase and pectinase, play a key role in biodegradation and hydrolysis, mechanisms of conspicuous importance in defence against invading pathogens, besides being decisive in getting nutrition from the host plant (Desire et al. 2014; Sunitha et al. 2013). These enzymes may act in harmonious orchestration with other pathogenesis-related proteins upon invasion of pathogens into the plant tissue or help in reducing phytopathogens directly degrading the cell wall of fungi and Oomycetes (Gao et al. 2010). Despite the fact that enzymes may not be fully effective as antagonistic agents, they may promote anta-gonistic activities if incorporated with other mechanisms. Simultaneously, different types of secondary metabolites are produced by the host plant through long-term co-evolution as a resistance mechanism to the established microorganisms including pathogenic and endophytic fungi. To defeat this obstruct, endophytic fungi must secrete the matching detoxification enzymes, such as cellulase, xylanase, and protease (Jia et al. 2016). Furthermore, enzymatic profiling may be used as an initial screen to select fungi capable of producing such proteins, and to provide preliminary information concerning the range of enzymes involved in the endophyte-host interaction to break down these secondary metabolites previous to the penetration across the defence systems of the inhabited host plants (Sieber 2007).
In the present research work, the crude extracts from the culture broth of endophytic fungi grown in PDB medium displayed antifungal activity that varied according to endophyte and pathogen. C. lunata (MF113056) showed the high inhibition capability (80%) of R. solani in vitro and of B. cinerea (48%). Antagonism might be due to the production of biologically active compounds in media. The detection of the antifungal activity in the crude extract of the isolate may indicate, but not prove, that this isolate produces bioactive substances. There are many reports about antimicrobial compounds produced by endophytes in cultures that were active against plant and human pathogenic microorganisms (e.g., Chareprasert et al. 2006; Kumar et al. 2014). A repository of secondary metabolites, possessing beneficial biological activities, may directly inhibit pathogens or induce systemic resistance to biotic stress factors (Fadiji and Babalola 2020). As stated by Kaaniche et al. (2019) Curvularia spp. secondary metabolites have captivated biological activities that compete with and antagonize other fungi by producing bioactive secondary metabolites with antifungal effects. In this investigation, phytochemical analysis of C. lunata (MF113056) crude extract revealed the existence of alkaloids and terpenoids as active secondary metabolites. It has been reported that endophytic fungi from the genus Curvularia, including C. trifolii (Samanthi et al. 2015) and C. lunata (Avinash et al. 2015), provide a wide assortment of bioactive secondary metabolites including polyketides and steroids which are well known for their different chemical structures and biological functions (Chathurdevi and Gowrie 2016). Likewise, Ramesha and Srinivas (2018) identified the presence of alkaloids and terpenoids in Curvularia sp. (RTFs-6) extract isolated from the medicinal plant Rauwolfia tetraphylla. Interestingly, the extract from Curvularia sp. IFB-Z10 displayed potent antimicrobial activity regarding the biosynthesis of curvulamine, an alkaloid (D’Costa et al. 2011). However, Parthasarathy and Sathiyabama (2013) reported the existence of terpenoids of the extract of endophytic fungus C. lunata isolated from Catharanthusroseus. Such bioactive metabolites belonging to alkaloids and terpenoids are well known for their antimicrobial activities (Boulogne et al. 2012; Ramesha and Srinivas 2014, 2018).
On the other hand, light microscope results showed that the crude extract of C. lunata (MF113056) obviously damaged the hyphal morphology of R. solani. The hyphae of R. solani produced heavily dark brown pigmented hyphae due to melanin production at the confrontation with the crude extract. The accumulation of melanin in fungi is needed for the fungus to overcome adverse environments such as protecting them from UV light, desiccation, toxic metals, and from the action of microbial lysis (Gessler et al. 2014). Mycelia of R. solani were completely collapsed and folding with the formation of short branches and undifferentiated tips. The morphological changes might result from the destruction of organelles in the endomembrane system due to the impact of the extract.
The positive results obtained from the in vitro studies encouraged the trials for utilizing C. lunata (MF113056) against R. solani causing root rot in faba bean in pot experiments. This was achieved by using different treatments: soaking faba bean seeds in filtrate of the antagonist; coating faba bean seeds with spores of the antagonist before sowing, as biological control involves the use of organisms and/or their products. As a result, the pot experiment clearly suggested that initial seed treatment with C. lunata (MF113056) either by filtrate or spore suspension would enhance faba bean tolerance to Rhizoctonia infection. The infection rates were significantly reduced by more than 50% when faba bean seeds were treated with filtrates and hyphal-spore suspension. Seed-soaking, root and stem dipping in filtrates of antagonistic organisms were effectively used for the control of various diseases (e.g., Hossain et al. 2016; Rustamova et al. 2020). While promising in scope, trials need to be conducted in natural systems to ascertain effectiveness in situ. Soil infected with R. solani and treated with C. lunata (MF113056) filtrates and hyphal suspension may exclude and reduce the build-up of fungal pathogens. It could be concluded from these results that C. lunata and its secondary metabolites had an inhibitory effect on the pathogen. Also, produced secondary metabolites may induce systemic resistance, leading to a higher level of host tolerance toward pathogens (Pieterse et al. 2014; Robert-Seilaniantz et al. 2011). Investigations on the mecha-nisms of disease suppression by endophyte extracts have suggested that the active principles present in them may either act on the pathogen directly, or induce systemic resistance in host plants resulting in reduction of disease development (Dutta et al. 2014; Pavithra et al. 2020).
The findings of the present study suggest the potential usage of the bioactive compounds of C. lunata (MF113056) to control plant fungal diseases but more studies on its effects and its bioproducts are needed. This potential crude extract of interest requires follow-up analysis, typically involving analytical chemists, and, further evaluations are needed for the biocontrol efficiency to plant diseases on the field level. The use of natural antifungal compounds rather than synthetic fungicides is of extraordinary interest. This paper opens unused-conceivable outcomes through the use of compounds obtained from natural sources for developing more secure techniques that may be used in plant disease management.
Parties annexes
REFERENCES
- Alori, E.T., and O.O. Babalola. 2018. Microbial inoculants for improving crop quality and human health in Africa. Front. Microbiol. 9: 2213.
- Anyasi, R.O. and H.I. Atagana. 2019. Endophyte: understanding the microbes and its applications. Pak. J. Biol. Sci. 22: 154-167.
- Avinash, K.S., H.S. Ashwini, H.N. Ramesh Babu, and Y.L. Krishnamurthy. 2015. Antimicrobial potential of crude extract of Curvularia lunata, an endophytic fungi isolated from Cymbopogoncaesius. J. Mycol. 2015: 185821.
- Bezerra, J.D.P., C.C.F. Nascimento, R.d.N. Barbosa, D.C.V. da Silva, V.M. Svedese, E.B. Silva-Nogueira, B.S., Gomes, L.M. Paiva, and C.M. Souza-Motta. 2015. Endophytic fungi from medicinal plant Bauhinia forficata: diversity and biotechnological potential. Braz. J. Microbiol. 46: 49-57.
- Bhardwaj, A., D. Sharma, N. Jadon, and P.K. Agrawal. 2015. Antimicrobial and phytochemical screening of endophytic fungi isolated from spikes of Pinus Roxburghii. Arch. Clinc. Microbiol. 6: 1-9.
- Boulogne, I., P. Petit, H. Ozier-Lafontaine, L. Desfontaines, and G. Loranger-Merciris. 2012. Insecticidal and antifungal chemicals produced by plants: a review. Environ. Chem. Lett. 10: 325-347.
- Busby, P.E., M. Ridout, and G. Newcombe. 2016. Fungal endophytes: modifiers of plant disease. Plant Mol. Biol. 90: 645-655.
- Chareprasert, S., J. Piapukiew, S. Thienhirun, A.J.S. Whalley, and P. Sihanonth. 2006. Endophytic fungi of teak leaves Tectona grandis L. and rain tree leaves Samanea saman Merr. World J. Microbiol. Biotechnol. 22: 481-486. doi:10. 1007/s11274-005-9060-x
- Chathurdevi, G., and S.U. Gowrie. 2016. Endophytic fungi isolated from medicinal plant-a promising source of potential bioactive metabolites. Int. J. Curr. Pharm. Res. 8: 50-56.
- D’Costa, V.M., C.E. King, L. Kalan, M. Morar, W.W.L. Sung, C. Schwarz, D. Froese, G. Zazula, F. Calmels, R. Debruyne, G.B. Golding, H.N. Poinar, and G.D. Wright. 2011. Antibiotic resistance is ancient. Nature 477: 457-461
- Deans, S.G., and G. Ritchie. 1987. Antibacterial properties of plant essential oils. Int. J. Food Microbiol. 5: 165-180. doi:10.1016/0168-1605(87)90034-1
- Desire, M.H., F. Bernard, M.R. Forsah, C.T. Assang, and O.N. Denis. 2014. Enzymes and qualitative phytochemical screening of endophytic fungi isolated from Lantana camara Linn. leaves. J. Appl. Biol. Biotechnol. 2: 1-6
- Dutta, D., K.C. Puzari, R. Gogoi, and P. Dutta. 2014. Endophytes: exploitation as a tool in plant protection. Braz. Arch. Biol. Technol. 57: 621-629.
- Fadiji, A.E., and O.O. Babalola. 2020. Elucidating mechanisms of endophytes used in plant protection and other bioactivities with multifunctional prospects. Front. Bioeng. Biotechnol. 8: 467.
- Food and Agriculture Organization of the United Nations. 2019. The state of food and agriculture 2019. Moving forward on food loss and waste reduction. Rome, Italy. 182 pp.
- Firáková, S., M. Šturdíková, and M. Múčková. 2007. Bioactive secondary metabolites produced by microorganisms associated with plants. Biologia. 62: 251-257. doi:10. 2478/s11756-007-0044-1
- Gao, F.-K., C.-C. Dai, and X.-Z. Liu. 2010. Mechanism of fungal endophytes in plant protection against pathogen. Afr. J. Microbiol. Res. 4: 1346-1351.
- Gessler, N.N., A.S. Egorova, and T.A. Belozerskaya. 2014. Melanin pigments of fungi under extreme environmental conditions (Review). Appl. Biochem. Microbiol. 50: 105-113.
- Hallmann, J., A. Quadt-Hallmann, W.F. Mahaffee, and J.W. Kloepper. 1997. Bacterial endophytes in agricultural crops. Can. J. Microbiol. 43: 895-914.
- Hankin, L., and S.L. Anagnostakis. 1975. The use of solid media for detection of enzyme production by fungi. Mycologia 67: 597-607. doi:10.2307/3758395
- Hossain, M.T., A. Khan, E.J. Chung, Rashid, M.H.-O., and Y.R. Chung. 2016. Biological control of rice bakanae by an endophytic Bacillus oryzicola YC7007. Plant Pathol. J. 32: 228-241.
- Jia, M., L. Chen, H.-L. Xin, C.-J. Zheng, K. Rahman, T. Han, and L.-P. Qin. 2016. A friendly relationship between endophytic fungi and medicinal plants: a systematic review. Front. Microbiol. 7: 906. doi:10.3389/fmicb.2016. 00906
- Kaaniche, F., A. Hamed, A.S. Abdel-Razek, D. Wibberg, N. Abdissa, I.Z. El Euch, N. Allouche, L. Mellouli, M. Shaaban, and N. Sewald. 2019. Bioactive secondary metabolites from new endophytic fungus Curvularia sp. isolated from Rauwolfia macrophylla. PLOS One 14: e0217627. doi:10. 1371/journal.pone.0217627
- Khan, A.L., J. Hussain, A. Al-Harrasi, A. Al-Rawahi, and I.-J. Lee. 2015. Endophytic fungi: resource for gibberellins and crop abiotic stress resistance. Crit. Rev. Biotechnol. 35: 62-74.
- Khan, A.L., R. Shahzad, A. Al-Harrasi, and I.-J. Lee. 2017. Endophytic microbes: a resource for producing extracellular enzymes. Pages 95-110 in D.K. Maheshwari, and K. Annapurna (eds.), Endophytes: crop productivity and protection. Springer, Cham, Switzerland.
- Khangura, R.K., M.J. Barbetti, and M.W. Sweetingham. 1999. Characterization and pathogenicity of Rhizoctonia species on canola. Plant Dis. 83: 714-721. doi:10.1094/PDIS. 1999.83.8.714
- Khare, E., J. Mishra, and N.K. Arora. 2018. Multifaceted interactions between endophytes and plant: developments and prospects. Front. Microbiol. 9: 2732 doi:10.3389/ fmicb.2018.02732
- Kumar, R., A. Sinha, S. Srivastava, and S. Singh. 2014. Evaluation of substrates for mass multiplication of green manure associated fungi for biological control of soil borne phytopathogens. Indian Phytopathol. 67: 396-401.
- Kusari, S., S. Singh, and C. Jayabaskaran. 2014. Biotechnological potential of plant-associated endophytic fungi: hope versus hype. Trends Biotechnol. 32: 297-303. doi:10. 1016/j.tibtech.2014.03.009
- Mejía, L.C., E.A. Herre, J.P. Sparks, K. Winter, M.N. García, S.A. Van Bael, J. Stitt, Z. Shi, Y. Zhang, M.J. Guiltinan, and S.N. Maximova. 2014. Pervasive effects of a dominant foliar endophytic fungus on host genetic and phenotypic expression in a tropical tree. Front Microbiol. 5: 479. doi:10.3389/fmicb.2014.00479
- Parthasarathy, R., and M. Sathiyabama. 2013. Screening and characterization of antimicrobial compound from endophytic fungus Curvularia lunata, isolated from Catharanthus roseus. World J. Pharm. Res. 2: 3078-3086.
- Pavithra, G., B. Sumant, R. Meenakshi, and S. Seweta. 2020. Role of endophytic microbes against plant pathogens: a review. Asian J. Plant Sci. 19: 54-62. doi:10.3923/ajps. 2020.54.62
- Pieterse, C.M.J., C. Zamioudis, R.L. Berendsen, D.M. Weller, S.C.M. Van Wees, and P.A.H.M. Bakker. 2014. Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 52: 347-375.
- Ramesha, A., and C. Srinivas. 2014. Antimicrobial activity and phytochemical analysis of crude extracts of endophytic fungi isolated from Plumeria acuminata L. and Plumeria obtusifolia L. Euro. J. Exp. Bio. 4: 35-43.
- Ramesha, A., and C. Srinivas. 2018. Antimicrobial activity and phytochemical analysis of endophytic fungal extracts isolated from ethno pharmaceutical plant Rauwolfia tetraphylla L. J. Pure Appl. Microbiol. 12: 317-332. doi:10. 22207/JPAM.12.1.38
- Robert-Seilaniantz, A., M. Grant, and J.D.G. Jones. 2011. Hormone crosstalk in plant disease and defense: more than just jasmonate-salicylate antagonism. Annu. Rev. Phytopathol. 49: 317-343.
- Rustamova, N., K. Bozorov, T. Efferth, and A. Yili. 2020. Novel secondary metabolites from endophytic fungi: synthesis and biological properties. Phytochem. Rev. 19: 425-448. doi:10.1007/s11101-020-09672-x
- Saad, M.M.G., R.Y. Ghareeb, and A.A. Saeed. 2019. The potential of endophytic fungi as bio-control agents against the cotton leafworm, Spodoptera littoralis (Boisd.) (Lepidoptera: Noctuidae). Egypt. J. Biol. Pest Co. 29: 7.
- Saikkonen, K., P. Wäli, M. Helander, and S.H. Faeth. 2004. Evolution of endophyte–plant symbioses. Trends Plant Sci. 9: 275-280.
- Samanthi, K.A.U., S. Wickramarachchi, E.M.K. Wijeratne, and P.A. Paranagama. 2015. Two new bioactive polyketides from Curvularia trifolii, an endolichenic fungus isolated from Usnea sp., in Sri Lanka. J. Natl. Sci. Found. 43: 217-224. doi:10.4038/jnsfsr.v43i3.7950
- Sieber, T.N. 2007. Endophytic fungi in forest trees: are they mutualists? Fungal Biol. Rev. 21: 75-89. doi:10.1016/j.fbr. 2007.05.004
- Sunitha, V.H., D.N. Devi, and C. Srinivas. 2013. Extracellular enzymatic activity of endophytic fungal strains isolated from medicinal plants. World J. Agric. Res. 9: 1-9. doi:10. 5829/idosi.wjas.2013.9.1.72148
- White, J.F., K.L. Kingsley, Q. Zhang, R. Verma, N. Obi, S. Dvinskikh, M.T. Elmore, S.K. Verma, S.K. Gond, and K.P. Kowalski. 2019. Review: endophytic microbes and their potential applications in crop management. Pest Manag. Sci. 75: 2558-2565.
Liste des figures
Figure 1
Fungal growth inhibition (%) of A. alternata, B. cinerea, F. oxysporum and R. solani by endophytic fungal filtrates using well diffusion technique
Figure 2
Figure 3
Suppression of Rhizoctonia root rot disease severity by endophytic fungi on faba bean: (a) Control (−) uninoculated plants. (b) Treated plants with C. lunata suspension. (c) Treated plants with C. lunata filtrates. (d) Control (+) inoculated plants with R. solani only. (e) Inoculated plants with R. solani and C. lunata suspension. (f) Inoculated plants with R. solani and C. lunata filtrates. Bar = 5cm.
Liste des tableaux
Table 1
Characteristics of the endophytes and the pathogenic fungi
Table 2
Endophytic fungi isolated from plant leaves and root and their ability to produce enzymes on solid media
Table 3
Preliminary qualitative screening of secondary metabolites of C. lunata (MF113056) filtrate
Table 4
Influence of filtrate and spore suspension of C. lunata on the incidence and severity of faba bean root rot disease under greenhouse condition