Résumés
Summary
A three-season field evaluation showed that eggplants (Solanum melongena) are very tolerant to damage caused by the two-spotted spider mite, Tetranychus urticae. Although large numbers of the predacious mites, Neoseiulus fallacis or Phytoseiulus persimilis were released, biological control of the two-spotted spider mite could not be sustained in the field. Based on a preliminary comparison of yield in both sprayed and unsprayed plots and on a three-year qualitative assessment, a tentative action threshold of 600 two-spotted spider mites per leaf would not decrease yield and would reduce the number of acaricide treatments per season. The new acaricide spirodiclofen was effective against the two-spotted spider mite in a preliminary trial.
Résumé
Une évaluation sur trois saisons a démontré que l’aubergine (Solanum melongena) est très tolérante au tétranyque à deux points, Tetranychus urticae. Malgré le grand nombre d’acariens prédateurs, Neoseiulus fallacis ou Phytoseiulus persimilis, relâchés au champ, la lutte biologique contre le ravageur n’a pu être maintenue au champ. Basé sur une évaluation préliminaire de la récolte dans les parcelles traitées et non traitées et sur une évaluation qualitative de trois années, un seuil provisoire de traitement de 600 tétranyques à deux points par feuille réduirait le nombre de traitements contre ce ravageur sans affecter la récolte. Le nouvel acaricide spirodiclofen a été efficace contre le tétranyque à deux points dans un essai préliminaire.
Corps de l’article
Introduction
In Quebec, eggplant, Solanum melongena L. is one of the more important minor vegetable crops produced by growers. An average of 42 metric t ha-1 are produced when grown under optimum conditions using plastic mulches and double row plantings (Anonymous 1992). This production is consistent from yr to yr and it provides growers with a steady income for their labour. In addition to the Colorado potato beetle, Leptinotarsa decemli-neata (Say), the two-spotted spider mite, Tetranychus urticae Koch [Acari: Te-tranychidae], has become one of the principal arthropod pests of eggplant. In recent yr, several strategies have been explored to manage T. urticae on eggplants. Sharaf (1984) reported that during the rainy month of January an Entomophtora fungus reduced T. urticae mite infestations and later in the season a Cecydomyiid (Feltiella sp.) was the principal predator in the Jordan valley. In Crete, Papadaki et al. (1985) reported the management of T. urticae with Phytoseiulus persimilis Athias-Henriot [Acari: Phytoseiidae] in plastic greenhouses. They concluded that the predator should be released at the first sign of T. urticae infestation in the greenhouse. Similar results were obtained by Chermiti (1991) again on eggplant in greenhouses, this time in Tunisia. Ethion, cyhalothrin, fenbutatin-oxide (Ho and Chen 1996), and abamectin (Chung et al. 2000) were found to be effective against T. urticae. Earlier, Patel et al. (1982) had shown that the use of carbaryl or endosulfan precipitated outbreaks of Tetranychus telarius L. in eggplants grown in the field. A similar observation was also made by Nemoto (1995), with imidacloprid causing Te-tranychus kanzawai Kishida resurgen-ces resulting from the destruction of natural enemies in eggplant fields in Japan. In Canada, malathion is the only pesticide registered to control T. urticae on eggplants. Since the acaricidal properties of this product are very limited (Anonymous 2001), this study was initiated to develop alternative management strategies including biological control, an empirical action threshold for treatment and a chemical alternative for malathion.
Materials and methods
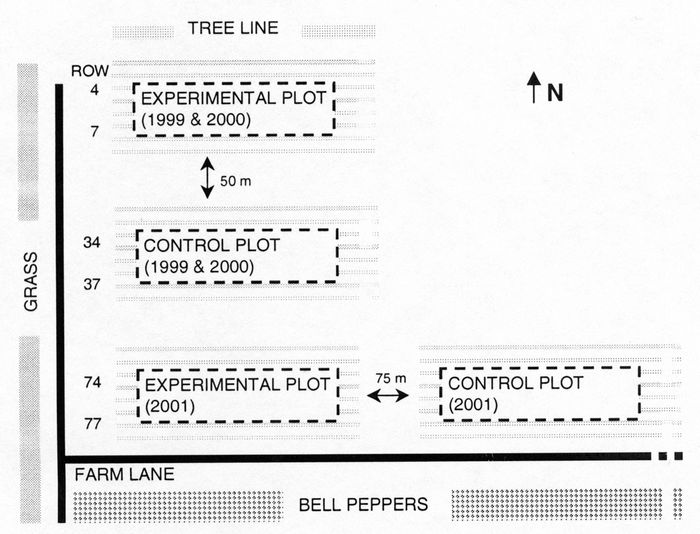
Field layout
The study was carried out in Saint-Remi (Quebec) from 1999 to 2001 inclusive. The experimental plot and control plot were part of a commercial field and each consisted of four double rows 20 m long, approximately 100 m2 (Fig. 1). The commercial field was 1.5 ha with double row plantings over a plastic mulch and drip irrigation underneath the mulch. The density of eggplants was 21 344 plants per ha. No acaricides were applied in the experimental plot except once in 2001 when T. urticae became very numerous (> 1000/leaf) where an oxydemeton-methyl (S-[2-(ethylsul-finyl)ethyl] O,O-dimethyl phosphorothioate) treatment was made at 840 g a.i. ha‑1. A control plot where only acaricides were used and no predators were released was established each yr to permit comparison of management techniques (Fig. 1). In 1999, the control plot received one treatment of abamectin (avermectin B1) on 22 July 1999 at 14.25 g a.i. ha‑1. In 2000 and 2001, it was treated respectively two and three times with oxydemeton-methyl at 840 g a.i. ha‑1. In 2000, the first treatment was made on 26 August and the second on 8 September. In 2001, the three treatments were respectively made on 21 and 29 July, and on 27 August 2001.
Figure 1
Field layout of the eggplant plots in Quebec, 1999, 2000 and 2001.
The plots were 20 m x 5 m. Neoseiulus fallacis (in 1999 and 2000) and P. persimilis (in 2001) were released in the experimental plot.
Mass rearing and release of predators
Neoseiulus fallacis (Garman) [Acari: Phytoseiidae] and Phytoseiulus persimilis were reared on T. urticae on bean plants according to Rock and Yeargan (1970). The N. fallacis were obtained from an ongoing culture that had originally been collected from a commercial orchard in Dunham, Quebec. These predators were known to be resistant to pyrethroid insecticides. The P. persimilis were obtained from Plant-Prod Québec (Laval, Quebec) and their numbers were increased several fold before their release in the field. Bean plants infested with few T. urticae and numerous predators were transported in coolers and distributed in the experimental plot. Number of predators released in the experimental plot were estimated by counting the number of predators on a sample of bean leaves using the washing-extraction method (Pratt and Croft 2000) and multiplying that figure by the average number of leaves distributed in the field on each release date. On average, 525 motile predators (350 to 700) were released on every third plant in the four rows at different intervals of time as reported in Figures 2 (A, B and C).
Evaluation of mite densities and crop yield
The abundance of T. urticae (1999, 2000 and 2001), N. fallacis (1999 and 2000) and P. persimilis (2001) was estimated by collecting leaves weekly and transporting them in paper bags placed in a cooler. Each leaf was placed in a separate paper bag. In 1999, because mass production of predators was delayed, regular weekly counts were implemented only in the latter part of the season. One medium-size leaf per plant was picked about 2/3 the height of the stem from 25 different plants, 1 m apart. Since these leaves were too wide to be brushed by the mite-brushing machine (Henderson and McBurnie 1943), they were dissected along the mid-rib and only one half of a leaf was brushed. Five half-leaves were brushed per glass plate for a total of five plates per plot per date. In 2001, a preliminary evaluation of yield was made comparing the eggplants produced by 15 plants from the experimental plot where predators had been released with those produced by 15 plants from the control plot. All fruit attaining marketable size (≌ 200 g) were counted, picked and weighed once a wk from 25 July to 5 September 2001.
Evaluation of spirodiclofen
A preliminary field evaluation of spirodiclofen (3-(2,4-dichlorophenyl)-2-oxo-1-oxaspiro[4.5]dec-3-en-4-yl 2,2-dimethylbutyrate), Envidor® 240 SC, Bayer Canada (Fischer and Benet-Buchholz 2002), a new acaricide not yet registered in Canada, to control T. urticae, was undertaken in 2001. The treatments were carried out with a battery-powered electric Dramm BP-502 (Manitowoc, WI. USA) back-sprayer set at 413.9 kPa. Five plants about 2 m apart were treated until runoff with 240 mg a.i. L‑1 of spirodiclofen. Each plant was considered as a replicate. Product effectiveness was established by estimating the number of mites found before and seven d after treatment on three leaves from the three inner plants; the two outer plants served as buffers. The control consisted of five plants treated with tap water and three leaves from the three inner plants were used for mite counts. The leaves were placed individually in separate paper bags and transported to the laboratory in a cooler. Mites were counted as described earlier. Variation between plots before treatment was reduced according to Bostanian et al. (1981), whereby the analyses of variance (ANOVA) was carried out on the ratio obtained by dividing mite counts after treatment by mite counts prior to treatment in the same plot. Ratios were then transformed to log (x + 2) and used for ANOVA.
Results and discussion
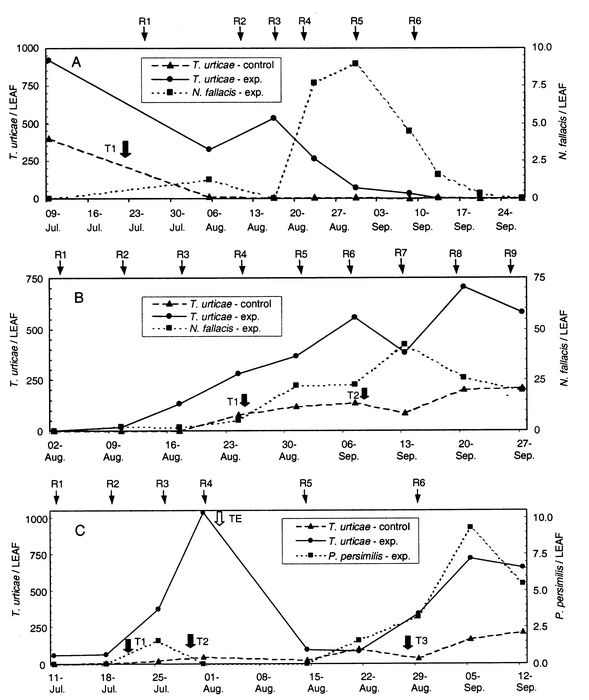
Results in Figure 2A show the population dynamics of T. urticae in relation to the presence of N. fallacis in 1999. Following an initial release of 60 000 predators on 26 July and subsequent releases weekly from 9 August, the population of the prey decreased from 917 T. urticae to four T.urticae per leaf by 13 September. Further scrutiny of Figure 2A shows a rapid increase in the number of N. fallacis following their release on 19 August. On 23 August the prey:predator ratio was 33:1 and by 30 August it was 18:1. On 8 September it decreased to 8:1 and by 13 September the prey density per leaf was close to zero. These results appeared encouraging in the development of a biocontrol alternative to manage T. urticae in eggplant.
Figure 2
A. Average number of T. urticae and N. fallacis per leaf on eggplants in Quebec, 1999. Neoseiulus fallacis releases (R) are shown with arrows (R1: 60 000; R2: 60 000; R3: 60 000; R4: 60 000; R5: 80 000 and R6: 80 000). A treatment with abamectin (T) was applied on 23 July in the control plot. B. Average number of T. urticae and of N. fallacis per leaf on eggplants in Quebec, 2000. Neoseiulus fallacis releases (R) are shown with arrows (R1: 60 000; R2: 80 000; R3: 80 000; R4: 100 000; R5: 100 000; R6: 240 000; R7: 120 000; R8: 100 000; R9: 100 000 and 3 releases (not shown) of 20 000 predators each were also made on prior dates: 14 June, 29 June and 26 July). Treatments in the control plot are indicated with arrows (T1 and T2: oxydemeton-methyl). C. Average number of T. urticae and P. persimilis per leaf on eggplants in Quebec, 2001. Phytoseiulus persimilis releases (R) are shown with arrows (R1: 80 000; R2: 120 000; R3: 160 000; R4: 180 000; R5: 80 000 and R6: 200 000. Treatments are indicated with filled arrows (T1, T2, and T3: oxydemeton-methyl) in the control plot and with an empty arrow (TE) in the experimental plot.
In apple orchards, Tanigoshi et al. (1983) reported that a generalized prey:predator ratio of 15:1 promoted biological control 90% of the time in Michigan. This ratio was based on extensive research of several scenarios for prey:predator ratios that had been evaluated by Croft and his associates in the seventies and early eighties. It considered the complex interactions between phytophagous and predacious mites in relation to weather, the cultivar and the growth stage of the host plant as well as pesticides used to control other pests than phytophagous mites. For example, certain apple cultivars such as ‘Red delicious’ were noted to be more nutritious to phytophagous mites than ‘MacIntosh’. Lush growth of the foliage in early season was also more nutritious than senescing foliage towards the end of the season. Finally, to control the other pests, selective pesticides that have minimal toxic effects to the predators had to be used. In Quebec, on average a prey:predator ratio of approximately 12:1 resulted in biocontrol of tetranychid mites by Amblyseius fallacis (Bostanian and Coulombe 1986).
In 2000, monitoring for T. urticae began earlier in order to release predators as soon as the buildup was recorded, rather than at maximum abundance of T. urticae as it was done in 1999. A total of 1 040 000 N. fallacis were released from 14 June to 27 September. N. fallacis attained maximum abundance of 42 predators per leaf and a prey density of 383 on 13 September (Fig. 2B). It is interesting to note that despite the release of 200 000 predators between 14 June and 10 August, the population of prey increased steadily until 7 September, and then it declined briefly. On 7 September the prey:predator ratio was 24:1 and then by 13 September it dropped to 9:1. However, for inexplicable reasons that ratio could not be maintained and the prey population increased while the predator population decreased. Prey density reached a maximum of 703 T. urticae per leaf while the predator density was 26 N. fallacis per leaf, bringing the prey:predator ratio to 27:1. In the control plot, T. urticae abundance attained 212 mites per leaf on 27 September (Fig. 2B) and no predators were found. This plot had been treated with oxydemeton-methyl on 26 August and 8 September.
After 2 yr of testing, biological control of the two-spotted spider mite by N. fallacis was at best marginal. Therefore, in 2001, P. persimilis, a relatively efficient mite predator in greenhouses (Papadaki et al. 1985), replaced N.fallacis in our study. The same experimental design and release tactics were used. A total of 820 000 P. persimilis were released from 11 July to the end of August (Fig. 2C). Despite the release of 540 000 P. persimilis between 11 July and 31 July, the population of the prey increased rapidly from 57 to 1036 motiles per leaf and an oxydemeton-methyl treatment was carried out on 2 August. By 14 August the prey density had decreased to 93 mites per leaf and another 80 000 P. persimilis were released. By August 21 the prey:predator ratio was 41:1 and another 200 000 predators were released on 28 August whereby the ratio of prey to predators began to tilt in favour of the predators. The predators attained a maximum abundance of nine predators per leaf on 5 September while the prey population was 717 mites per leaf. The last count was made on 12 September when the predator density had decreased to five and the pest density had decreased to 657 per leaf. In the control plot three treatments of oxydemeton-methyl had been applied on 21 July, 29 July and 27 August. Comparatively, the phytophagous mite density remained relatively low in this plot, although it eventually increased to 214 mites per leaf at the end of the season (Fig. 2C). No predators were found in this control plot throughout the entire season.
Figure 3 reports the yield and the population dynamics of T. urticae in the experimental plot and the chemically treated control plot in 2001. We note that at the beginning of the season the plot where the predators had been released produced more eggplants than the control plot. However, as the season advanced this difference decreased and by the sixth eggplant pick, the total weight of eggplants from either plot was very similar. This means that eggplants are extremely tolerant to mite infestations for even at 1 036 mites per leaf (1 August, Fig. 3), the yield was not adversely affected. However, at such high densities T. urticae were noted to be crawling on the calyx and the fruit itself and washing the crop became a requirement before marketing. When mite density did not exceed 700 mites per leaf (5 September, Fig. 3), no washing was required, saving this timely and costly operation.
Figure 3
Average number of T. urticae per leaf and eggplant yield in total kilograms of fruit produced by 15 plants in both the acaricide treated and the experimental plots in Quebec, 2001.
From a scientific perspective, biological control of the two-spotted spider mite by N. fallacis or P. persimilis that would be acceptable by the grower community was attained only once with N. fallacis during this study. We believe that the exponential increase of two-spotted spider mite populations on eggplants make it virtually impossible for any predator to increase its numbers sufficiently to decrease the two-spotted spider mite populations to lower densities. This may be exacerbated by lush growth of eggplants thanks to drip irrigation accompanied by hot and dry summers.
From an economic perspective, the costs would never be acceptable by the grower community using current agronomic activities. Projecting predator requirements based on the results of 1999, when N. fallacis managed to control the two-spotted spider mite, we would need an average of 40 x 106 predators per ha. The cost of so many predators would be $733 920 (Plant-Prod Québec, Laval, Quebec). Phytoseiulus persimilis which was even less effective than N. fallacis (Fig. 2C), would cost even more on a ha basis ($1 343 160). When it became apparent that biological control would not be feasible, the search for a more effective acaricide than malathion was undertaken in 2001.
Figure 4 summarizes the preliminary results of managing T. urticae with spirodiclofen. This acaricide is an inhibitor of lipid biosynthesis in mites. Seven d after treatment, we recorded a highly significant decrease in egg numbers (F = 71.1; df = 1; P = 0.01) as well as the motile forms (F = 96.2; df = 1; P= 0.01). These results suggest that spirodiclofen is effective against T. urticae adults and eggs on eggplants just as it was effective against the European red mite, Panonychus ulmi on apples (Bostanian and Racette 2002).
Figure 4
Average number of T. urticae eggs (top) and motiles (bottom) per leaf on eggplants before and after treatment (TRMT) with spirodiclofen.
Note: Bars with the same letters at each treatment date are not significantly different at alpha = 0.05, Duncan’s multiple range test.
In synopsis, the results of this study indicate that biological control of T. urticae on eggplants is economically non-feasible even though eggplants have a very high tolerance to T. urticae. On a ha basis, the number of predators required would be stupendous and the cost prohibitive. On the other hand, it would be possible to reduce the number of acaricide applications as the eggplant tolerates a high density of T. urticae without any decrease in yield. In this respect, we propose 700 mites per leaf as a tentative empirical action threshold based on our results that should be verified by additional studies. However, as this preliminary thres-hold may be attained during adverse weather conditions and it may not be possible to enter and treat the field promptly, a 600 mite per leaf action threshold may be more appropriate. Thus, a buffer of 100 mites per leaf would provide the time span for the return of better weather conditions for an acaricide treatment. Spirodiclofen is a very effective ovicide and adulticide of T. urticae and additional field evaluations should be carried out to develop the data required to replace malathion.
Parties annexes
Remerciements
The authors gratefully acknowledge the cooperation of the eggplant grower, Mr Clement Oligny. This project was financed by an Agriculture and Agri-Food Canada MII project (# 99-5915) granted to the research team. Agriculture and Agri-Food Canada, contribution # 335/2003.03.02R.
References
- Anonymous. 1992. Légumes en terre minérale. C.R.E.A.Q. Agdex 250/821. MAPAQ, Quebec (Quebec). 43 pp.
- Anonymous. 2001. Avertissement : Cucurbitacées-Solanacées. Réseau d’avertissements phytosanitaires. No 7, 29 June 2001, MAPAQ, Quebec (Quebec). 6 pp.
- Bostanian, N.J., and L.J. Coulombe. 1986. An integrated pest management program for apple orchards in southwestern Quebec. Can. Entomol. 118 : 1131-1142.
- Bostanian, N.J., and G. Racette. 2002. Evaluation of BAY BAJ 2740 240 SC to manage phytophagous mites in apple orchards 1999, 2000 and 2001. Arthropod Manag. Tests 27 : Reports # A6, A7 and A8.
- Bostanian, N.J., R.O. Paradis, and D. Pitre. 1981. Susceptibility of phytophagous mites to a single summer treatment of acaricides in a Quebec apple orchard. Phytoprotection 62 : 33-38.
- Chermiti, B. 1991. Lutte biologique : 1-. Essai d’utilisation de Phytoseiulus persimilis Athias-Henriot (Acarina, Phytoseiidae) contre Tetranychus urticae Koch (Acarina, Tetranychidae) sur une culture protégée d’aubergine. Bull. O.I.L.B./S.R.O.P. Lutte intégrée en cultures protégées-climat méditerranéen. Allasio, Italy. Pages 134-139.
- Chung, B.K., S.W. Kang, and J.H. Kwon. 2000. Chemical control system of Frankliniella occidentalis (Thysanoptera: Thripidae) in greenhouse eggplant. J. Asia-Pacific Entomol. 3 : 1-9.
- Fischer, R., and J. Benet-Buchholz. 2002. Chemistry and stereochemistry of spiro-diclofen (BAJ 2740). Pages 137-148 in M. Esters (ed.), Pflanzenschutz Nachrichten Bayer. Decker Druck, Leverkusen, Germany.
- Henderson, C.F., and H.V. McBurnie. 1943. Sampling technique for determining populations of the citrus red mite and its predators. U.S. Dep. Agric. Circ. 671.
- Ho, C.C., and W.H. Chen. 1996. Improvement on the chemical control of spider mites of eggplant. J. Agric. Res. China 45 : 285-296.
- Nemoto, H. 1995. Pest management systems for eggplant arthropods: a plan to control pest resurgence resulting from the destruction of natural enemies. Jpn Agric. Res. Q. 29 : 25-29.
- Papadaki, M.E., T.J. Fitsakis, and E.C. Kozirakis. 1985. Integrated control of greenhouse whitefly and the red spider mite in Crete. Bull. O.I.L.B./S.R.O.P. Integrated control in protected crops. Catania, Italy. Pages 19-26.
- Patel, C.B., A.H. Shah, and S.H. Patel. 1982. Effect of insecticides on the resurgence of two-spotted spider mites, Tetranychus telarius Linn., in brinjal. Indian J. Agric. Sci. 52 : 774-776.
- Pratt, P.D., and B.A. Croft. 2000. Overwintering and comparative sampling of Neoseiulus fallacis (Acari: Phytoseiidae) on ornamental nursery plants. Environ. Entomol. 29 : 1034-1040.
- Rock, G.D., and R.R. Yeargan. 1970. Relative toxicity of plictran to the European red mite, the two-spotted spider mite and the predaceous mite Neoseiulus (Typhlodromus)fallacis (family: Phytoseiidae). Down Earth 26 : 1-4.
- Sharaf, N.S. 1984. Studies on natural enemies of Tetranychid mites infesting eggplant in the Jordan valley. Z. Angew. Entomol. 98 : 527-533.
- Tanigoshi, L.K., S.C. Hoyt, and B.A. Croft. 1983. Basic biology and management components for mite pests and their natural enemies. Pages 153-202 in B.A. Croft and S.C. Hoyt (eds), Integrated management of insect pests of pome and stone fruits. John Wiley & Sons, NY.
Liste des figures
Figure 1
Field layout of the eggplant plots in Quebec, 1999, 2000 and 2001.
Figure 2
A. Average number of T. urticae and N. fallacis per leaf on eggplants in Quebec, 1999. Neoseiulus fallacis releases (R) are shown with arrows (R1: 60 000; R2: 60 000; R3: 60 000; R4: 60 000; R5: 80 000 and R6: 80 000). A treatment with abamectin (T) was applied on 23 July in the control plot. B. Average number of T. urticae and of N. fallacis per leaf on eggplants in Quebec, 2000. Neoseiulus fallacis releases (R) are shown with arrows (R1: 60 000; R2: 80 000; R3: 80 000; R4: 100 000; R5: 100 000; R6: 240 000; R7: 120 000; R8: 100 000; R9: 100 000 and 3 releases (not shown) of 20 000 predators each were also made on prior dates: 14 June, 29 June and 26 July). Treatments in the control plot are indicated with arrows (T1 and T2: oxydemeton-methyl). C. Average number of T. urticae and P. persimilis per leaf on eggplants in Quebec, 2001. Phytoseiulus persimilis releases (R) are shown with arrows (R1: 80 000; R2: 120 000; R3: 160 000; R4: 180 000; R5: 80 000 and R6: 200 000. Treatments are indicated with filled arrows (T1, T2, and T3: oxydemeton-methyl) in the control plot and with an empty arrow (TE) in the experimental plot.
Figure 3
Average number of T. urticae per leaf and eggplant yield in total kilograms of fruit produced by 15 plants in both the acaricide treated and the experimental plots in Quebec, 2001.
Figure 4
Average number of T. urticae eggs (top) and motiles (bottom) per leaf on eggplants before and after treatment (TRMT) with spirodiclofen.