Abstracts
Abstract
The development of molecular tools for agricultural analysis promoted property crop production improvement to rising food security from stress caused by organic phenomenon factors, as well as the insect pest attack. Crop productivity has inflated quintuple over the past few decades due to high-yielding varieties, irrigation, fertilizers, and pesticides. However, the planet population is anticipated to grow staggeringly over subsequent four decades, from nine to ten billion individuals. It’s so imperative to extend the assembly of food grains to feed the population. As way as ancient agriculture technology is bothered, it still incorporates a good distance to travel. The molecular tools wont to shield crops from organic phenomenon stress during this instance like plant alpha-amylase inhibitors, vacuolar ATPase, hemoprotein P450 monooxygenase, enzyme modulating oostatic issue (TMOF), enzyme inhibitors (PIs), with the exception of sterol enzyme (CHOx), lipoxygenases (enzyme), and technology, etc. despite not being wholesome. Additionally to delivery ecologically property farming practices into our daily lives, bionanotechnology will give opportunities for developing nations and developed nations shortly. It should have an enormous impact on farming systems while not the employment of pesticides. There area unit considerations concerning the food safety risks related to transgenic plants since the unfold of antibiotic resistance, the changes within the nutrient composition of plants, and also the production of noxious proteins and allergens can not be answered currently. Besides victimization high-yielding varieties, irrigation, and fertilizers, it’s a potent role in minimizing the economic losses caused by insects. Within the current context of insect pest management, it’s imperative to develop bionanotechnology-based tools for pest management. However, their economical use necessity takes for reckoning the numerous ecological role useful insect play for the longer-term development of agriculture.
Keywords:
- food security,
- insect pest management,
- molecular tools,
- yield
Résumé
Le développement d’outils moléculaires pour l’analyse agricole a favorisé l’amélioration de la production des cultures afin d’accroître la sécurité alimentaire face aux stress causés par des phénomènes organiques ou par des attaques d’insectes nuisibles. La productivité des cultures a quintuplé au cours des dernières décennies grâce aux variétés à haut rendement, à l’irrigation, aux engrais et aux pesticides. Cependant, la population de la planète devrait connaître une croissance vertigineuse au cours des quatre prochaines décennies, passant de neuf à dix milliards d’individus. Il est donc impératif d’étendre la disponibilité des céréales alimentaires pour nourrir la population. Comme la technologie agricole ancienne est perturbée, il lui reste encore un long chemin à parcourir. Les outils moléculaires ont l’habitude de protéger les cultures de stress liés aux phénomènes organiques, comme les inhibiteurs de l’alpha-amylase, l’ATPase vacuolaire, l’hémoprotéine P450 monooxygénase, l’enzyme modulant le problème oostatique (TMOF), les inhibiteurs d’enzymes (IP), à l’exception de l’enzyme stérol (CHOx), les lipoxygénases (enzymes), et la technologie, etc., bien qu’ils ne soient pas sains. En plus d’apporter des pratiques agricoles écologiques dans notre vie quotidienne, la bionanotechnologie ouvrira sous peu des opportunités aux pays en développement et aux pays développés. Elle devrait avoir un impact énorme sur les systèmes agricoles tout en évitant l’emploi de pesticides. Il existe des considérations à propos des risques de sécurité alimentaire liés aux plantes transgéniques, étant donné que le développement de la résistance aux antibiotiques, les changements dans la composition nutritive des plantes et la production de protéines nocives et d’allergènes n’ont pas encore été évalués. Outre le recours à des variétés à haut rendement, à l’irrigation et aux engrais, cela joue un rôle important dans la réduction des pertes économiques causées par les insectes. Dans le contexte actuel de lutte intégrée contre les ennemis des cultures, il est impératif de développer des outils de lutte basés sur les bionanotechnologies. Cependant, leur utilisation économique nécessite de prendre en compte les nombreux rôles écologiques que jouent les insectes dans le développement à long terme de l’agriculture.
Mots-clés :
- lutte intégrée,
- outils moléculaires,
- rendement,
- sécurité alimentaire
Article body
INTRODUCTION
World population area unit increasing day by day staggeringly, thus agricultural production is additionally rising within the same thanks to feed them. Additionally, we have a tendency to conjointly achieve some landmarks in cereal production by victimization of new varieties, fertilizers, and pesticides. Pesticides aren't forever best since they are not eco-friendly. After that, the Integrated Pest Management (IPM) system approach confers commercial enterprise production and pest management practices to attenuate the economic losses caused by pests. Several plant protection specialists developed multi-tactical methods for dominant pests within the early twentieth century, wishing on information of pest biology and cultural practices (Gaines 1957). Observation causes and protective the pests' natural enemies area unit the key parts of pest management. As an environmentally safe and cost-efficient management technique for a large vary of inver-tebrate pests, insect pathogens befell confirmed to be effective. Today, pest-associated losses makeup a minimum of Bastille Day of the overall agricultural production (Oerke 2006) and may diminish by minimizing pest-associated losses (H.C. Sharma and Rao 1995).
A massive application of pesticides to attenuate losses thanks to insect pests, diseases, and weeds has resulted in an exceedingly high chemical residue in food and food product, which harms useful organisms within the setting. Presently an outsized variety of insect species area unit immune to presently offered pesticides, that has needed the applying of upper doses or a lot of frequent application of pesticides. Crop advancement has created the “Green Revolution” one in all the assorted important scientific shifts in social science history. Rice, wheat, and maize production have widened undeviatingly over the past decade, surpassing the past century’s accomplishments (Swaminathan 2000). The use of advanced mechanisms of biological science to pest oversight will considerably enhance food production. Crop production and protection and its sub-components should be positively interconnected (Fig. 1). Environmental and climate change should not affect the impact of which.
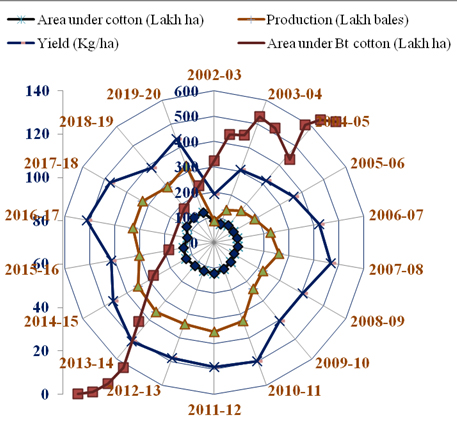
Transgenic insect-resistant crops have done extensively exhibited prompting various considerations concerning their interactions with non-target organisms within the setting, the biosafety of food derived from genetically designed crops, and their environmental impact. Cotton and Bt cotton area under cultivation in India augmented from 2002-03 to 2019-20 (Fig. 2) due to technological advances and better farm management practices. Insect pests are often managed by victimization natural enemies, biopesticides, natural plant product, and pest-resistant varieties. Thus, molecular biological tools are often wont to management insect pests, like alpha-amylase inhibitors of plants (Franco et al. 2002), hemoprotein-P450 monooxygenase, vacuolar ATPase (Mao et al. 2007), enzyme modulating oostatic issue (TMOF) (Tortiglione et al. 2002), enzyme inhibitors (PIs) (Tamhane et al. 2007), with the exception of sterol enzyme (CHOx) (Shukle and Murdock 1983), lipoxygenases catalyst (Felton et al. 1994), etc., may play polar roles here. To boot, biological science involves victimization recombinant DNA technology to develop stronger natural enemies and microbes a lot of virulent than natural enemies. The treatise centered on the safety of genetically changed plants, the detection of transgenic(s) in food and food product, and also the applications of recent biotechnology to pest management and property agriculture. As food security continues to be vulnerable by organic phenomenon stress, at a time once various maneuvers area unit desperately required, this chapter is especially relevant. Researchers, scientists, NGOs, directors, and students can all enjoy this technology within the 21st century.
Figure 1
Interlinking of domains
Figure 2
Year-wise area under cotton, Bt cotton, production and yield of cotton during 2002-03 to 2019-20
Molecular approach to insect pest management
For economic process to continue for the predictable future, productivity gains area unit essential. However, within the short run, they’re even a lot of imperative for maintaining food provides. The appraisal and sensible analysis of molecular tools which implicated for increasing crop production area unit crucial. Molecular biological tools area unit utilized in crop improvement and so plant alpha-amylase inhibitors use for insect pest management (Franco et al. 2002). vacuolar ATPase, hemoprotein P450 monooxygenase (Mao et al. 2007), enzyme modulating oostatic issue (TMOF) (Tortiglione et al. 2002), enzyme inhibitors (PIs) (Tamhane et al. 2007), with the exception of sterol enzyme (CHOx) (Shukle and Murdock 1983), lipoxygenases catalyst (Felton et al. 1994), etc., have tremendous roles. Among the essential amino acids area unit those that enhance time period and postharvest quality, enhance chemical element uptake and fixation, combat soil salinity, and aluminum toxicity, improve chemical action, sugar production, starch yield, and crop yield, and turn out antibodies, prescription drugs, and vaccines. With the introduction of recent crop cultivars that area unit immune to insect pests and diseases, along-side biocontrol agents, it ought to be potential to cut back the necessity for pesticides among farmers whereas benefiting each the setting and public health.
Plant catalyst matter in insect pest management
Enzymatic inhibitors act on the insect gut organic process enzymes, like alpha-amylases and proteinases, which play a vital role within the digestion of plant starch and proteins. The treatments conjointly embody lectin-like and knottin-like inhibitors, cereal-type inhibitors, Kunitz inhibitors, gamma-purothionin inhibitors, and thaumatin inhibitors (Franco et al. 2002). Introducing an alpha-amylase matter into a transgenic pea, Phaseolus vulgaris L. prevented the seed weevil larvae from developing. Alpha-amylase inhibitors defend plants from predators, as well as bruchids, Lacanobia oleracea (L.), Bruchus pisorum (L.) in transgenic peas. Amaranth seeds inhibited alpha-amylase activity in Tecia solanivora (Povolny) larvae (Valencia-Jimenez et al. 2008).
A K+ pump creates electrical variations in midgut cells that alter nutrient uptake. Besides, the K+ pump dominates the hydrogen ion concentration of the midgut lumen and circumscribes the atomic number 19 consistency in blood, animal tissue cadres, and midgut lumen. A nucleon ATPase of the vacuolar-type is that the primary motor for transport. As an example, transgenic corn sprouts exposing dsRNA of a V-ATPase from western corn rootworm showed a major decrease in corn rootworm feeding and harm to plants. The mono-oxygenase part of hemoprotein-P450 equips insects to tolerate oppositely restrictive solidities of gossypol, a cotton substance. As an example, Helicoverpa armigera (Hübner) ate up plants revealing hemoprotein-P450 dsRNA grew more well-off. Mao et al. (2007) found that gossypol considerably restrained growth. Among the category of plant defence proteins those stop insects pest infestations area unit enzyme inhibitors. By inhibiting endogenous enzymes, it’s potential to argue that they shield plants against insects and presumably pathogens (K. Sharma 2015). In direct response to mechanical wounds and bug attacks, plants turn out enzyme inhibitors constitutively and induced enzyme inhibitors. The vapours discharged by these plants may attract predators of insect herbivores once insect harm. Uninjured plants factory-made protease inhibitors being a consequence of the liberation of animated syntheses once gashing, like alkyl jasmonate-cotton substance, creating them a lot of immune to insects. Adding enzyme inhibitors to substitute nutrition or expressing them in transgenic manufactories lead to inflated mortalities among insects, significantly those happiness to animal order, Orthoptera, and Lepidoptera (Tamhane et al. 2007).
Trypsin-modulating oostatic inhibit the maturity and construction of dipterous insect larvae death by starvation for larvae that kill them (Lau et al. 2011). The amide blocks the synthesis of enzyme in mosquitoes, Aedes aegypti (L.), and flesh flies. As an example, vaccination or verbal bodily process of Aea-TMOF pissed off enzyme synthesis and larval completion in Heliothis virescens (Fabricius). Tortiglione et al. (2002) found H. virescens mortality inflated with Aea-TMOF-expressing transgenic tobacco plants. Lipoxygenases catalyst created from plants might play a job within the host's resistance against insect pests. Unsaturated fatty acids area unit hydroperoxides created by dioxygenase enzymes in plants that harm insect midgut membranes. Lipoxygenase from soybeans, as an example, impedes the adulthood of Manduca sexta (L.) once glued to an imitation diet (Shukle and Murdock 1983). Treatments reduced Helicoverpa zea (Boddie) larvae growth by 24% to 63% (Felton et al. 1994).
Cholesterol enzyme (CHOx) is an enzyme that catalyzes the chemical reaction of sterol. Sterol enzyme (3β-hydroxysteroid enzyme, EC 1.1.3.6) created by each morbific and nonpatho-genic microorganisms, as well as mycobacteria, Brevibacterium, actinomycete, eubacteria, Arthrobacter, Pseudomonas, Rhodococcus, Chromobacterium, and Bacillus species (Devi and Kanwar 2017). Peroxide is created from sterol and alternative 3-hydroxysterols by this microorganism catalyst, and also the corresponding 3-hydroxysterols and midgut membranes broken because the principal perform. As an example, the sterol enzyme from actinomycete caused acrobatics of H. virescens, H. zea, and Pectinophora gossypiella (Saunders) once consolidated into an artificial nutrition. Consolidating tobacco leaves expressing sterol enzyme into artificial diets prompted extinction and determined acrobatics in baby boll weevil larvae (Corbin et al. 2001). Immunity in insects begins with the basic cognitive process of microbic infection. Microbic pattern recognition proteins, like hemolin, area unit coupled to causation this protecting result. As an example, hemolin ribonucleic acid was injected into lepidopterous insect pupae of large silkmoths, Hyalophoracecropia (L.) and also the moths developed frequently. The eggs with deformed embryos didn't turn out larvae once sexual activity (Eleftherianos et al. 2006). It absolutely was determined that previous unwellness of M. sexta larvae with a nonpathogenic Escherichia coli T. Escherich, angry protection versus ensuant contamination as well as the deadly micro-organism Photorhabdus luminescens (Thomas et Poinar). An insect injected with hemolin ribonucleic acid was likewise sensitive to P. luminescens infection than an insect that had not antecedently been infected with E. coli physiology differs between insect species. An insect organic process PI prevents supermolecule digestion by binding to insect proteases preventing chemical action. By under nourishing the larvae of super molecules and indispensable amino acids, it frustrates their completion and development. Examples, mouse-ear cress serPin-1 reduced genus Spodoptera littoralis (Boisduval) biomass by 38% once four days of feeding, barley enzyme matter reduced genus Spodoptera exigua (Hübner) survival by 29%, bovine spleen enzyme matter reduced H. armigera growth (Christeller et al. 2002), and cowpea enzyme matter shrunken mortality in H. armigera and H. virescens.
Multivalent carbohydrates plight to midgut animal tissue blocks, inhibiting their gathering, interrupting nutrient transmission, and starting in all probability corrosive parts to be assimilated. As an example, soybean seed glycoprotein inhibited the expansion of M. sexta. Agglutinins from aliment were noxious once fed to Ostrinia nubilalis (Hübner). Peritrophic membranes were discontinuous within the anterior midgut microvilli. Antibody (WGA) inhibited powerfully the expansion of O. nubilalis, however not that of M. sexta. Once disclosed to WGA, O. nubilalis larvae caused extraordinarily disguised polymers within the anterior midgut lumen and destroyed microvilli, consecutive showing in no a lot of prolonged feeding. The event of L. oleracea larvae was reduced by transgenic potatoes expressing Anemone quinquefolia (L.) glycoprotein. S. littoralis larvae were dim-witted in transgenic tobacco plants expressing leaf (ASAL) and bulb (ASAII) agglutinins from garlic. Mediterranean flour moth larval growth qualified by Moringa oleifera lectin (cMoL) consolidated into artificial nutrition. Immature extinction extended thanks to increased evolution past.
Vegetative insecticidal protein (Vip)
Insect-pathogenic microorganisms like Bacillus cereus dicot genus and B. thuringiensis Berliner secrete insecticidal proteins that accumulate in crystals and inclusion bodies. The plantative insecticidal proteins act as binary toxins against lepidopteran, coleopteran and hemipteran insects. Its supermolecule binds to receptors on the midgut membrane and enters the cell wherever it inhibits microfilament formation by showing its ADP-ribosyl enzyme activity (Chakroun et al. 2016). A Vip-treated animal of Agrotis ipsilon (Hufnagel), Spodoptera frugiperda Smith, Helicoverpa zea (Boddie), Trichoplusia ni (Hübner), Plutella xylostella (L.), Pieris brassicaewere (L.) paralytic, had his animal tissue cells utterly lysed, and died (Sellamiet et al. 2011).
Biosafety evaluations and eco-friendliness enclosed in genetically changed crops (GM). A world increase in GM cotton crop space and production has occurred from 1.7 million hectares in 1996 to 191.7 million hectares in 2018 (Government of India 2020). A complete of 99 of the worldwide yield comes from five major biotech crops – soybeans, maize, cotton, canola, and alfalfa (English and Schreiber 2020). Whereas some insect populations have evolved resistance to Bt proteins, Bt transgenics are widely used for insect pest management, in the main to manage lepidopterous insects. It’s been shown that the corn insecticidal supermolecule, IPD072Aa, derived from Pseudomonas chlororaphis (Guignard and Sauvageau), confers resistance to western corn rootworm (WCR), that may be a damaging crop pest in North America and in Europe. Though it’s effective against some Lepidoptera and order Hemiptera (Schellenberger et al. 2016), it is not effective against all of them.
At one purpose in time, 22 crops from 41 countries were approved, grown, and commercialized as genetically designed (GE) crops. The suspension of some cultivation has occurred. The grayed-out countries now not grow GE crops (Fig. 3).
Figure 3
Global area of biotech crops, 1996 to 2018
Compounds in winter melon (HVC)
Honeydew contains organic acids, amino acids, and lipids (Hussain et al. 1974; Leroy, Wathelet et al. 2011; Mittler 1958) secreted by consumption insects like aphids. Additionally to its use as a food supplement (Hogervorst et al. 2007; Lee et al. 2004; Wackers 2000), it acts as a volatile substance for parasitoids and predators (Almohamad et al. 2009; Budenberg et al. 1992; Buitenhuis et al. 2004; Choi et al. 2004; Du et al. 1997; Scholz and Poehling 2000; Verheggen et al. 2008). Microorganisms will turn out semiochemicals that act as associate attracting or replanting agent for the natural enemies of various insects (Epsky et al. 1998; Robacker and Lauzon 2002; Robacker et al. 1998). Solely a number of studies have examined whether or not microorganism area unit able to turn out volatile compounds (Bunge et al. 2008; Kai et al. 2009; Schulz and Dickschat 2007). In some studies, two microorganisms were isolated from the gut of pea aphids, Staphylococcussciuri and within the winter melon flora of untamed aphids, Acinetobacter calcoaceticus, that enhance louse natural enemies’ effectiveness (Harad et al. 1996; Haynes et al. 2003; Leroy, Sabri et al. 2011).
Nanotechnology and insect pest control
Nanotechnology and bug tormenter management presently, engineering science, is getting used as a tool for crop protection and tormenter management. Nanoparticles are also used as carriers or as pesticides or pesticides. By making many sorts of natural resource-based virulent materials victimization engineering science, we are able to play a very important role in crop protection.
A nanomolecular polymer
Globalization has brought new dimensions, innovations, and changes in plant protection technology. Animals, humans, and also the settings are considerably improved with new steps in agricultural security. As a results of advances in plant protection and new developments in engineering science, crops everywhere the globe area unit protected against insects and diseases. Additionally to developing new nano-materials, engineering science also can facilitate to provide new insect tormenter resistant varieties by written material and the genetic makeup of crops varieties (Gopal et al. 2012; Savary et al. 2006; Sparks et al. 2012). For instance, some vital chemical compound typically utilized in the nanoparticles production viz., anionic surfactants (Elek et al. 2010); carboxymethyl chitosanricinoleic acid (Azadirachtin) (Feng and Peng 2012); lignin-polyethylene glycol-ethylcellulose and chitosan-poly(lactide) (Imidacloprid) (Flores-Cespedes et al. 2012; Li et al. 2011); polythene (Piperonyl, Butoxide and Deltamethrin) (Frandsen et al. 2010); acrylic acid-buacrylate (Itraconazole) (Goldshtein et al. 2005); polymer, vinylethylene and vinylacetate (Pheromones) (Hellmann et al. 2011; Wright 1997); alginate-glutaraldehyde (neem seed oil) (Kulkarni et al. 1999); N-(octadecanol-1-glycidyl ether)-O-sulfate chitosan with octadecanol glycidyl ether (rotenone) (Lao et al. 2010).
Gold nanoparticles
The wonderful physical and chemical properties of the neon-technology change their use nowadays in medicine, environ-mental, and biological analyses. A. aegypti, genus Anopheles stephensi Liston, arthropod genus quaccifeias, Benelli (2018), and arthropod genus S. litura area unit a number of the vectors that area unit managed with gross national product nowadays (Chakravarthy et al. 2012; Chandrashekharaiah et al. 2015) in zoology.
Chitosan and agrochemical loaded nanoparticles
Agrochemicals were loaded (spinosad and permethrin) into the chemical compound nanoparticles created by A. Sharma et al. (2019). Perishable, non-toxic, biocompatible, and adsorbent, chitosan has glorious sorption properties. As check ways for survivability and climb of Drosophila melanogaster Meigen and arthropod genus litura, agrochemical-loaded chitosan nano formulations were the foremost effective (Chandra et al. 2013). Chinese corn borer, which ends in death, was spellbound to a fluorescent nanoparticle that supported genetic management (Bicheng et al. 2013).
Silver nanoparticles (AgNPs)
Some researchers have reported the medication, antiviral, and antifungal properties of silver nanoparticles (Panáček et al. 2009; Santhoshkumar et al. 2011; Shankar et al. 2004) and additionally, leaf extract from dicot genus indica has antifeedant, larvicidal, and cytotoxic properties against H. armigera (Vendan et al. 2009). These AgNPs were accustomed management the adults of Sitophilus oryzae (L.), Haemaphysalis bispinosa Neumann and Hippobosca maculate (Zahir and Rahuman 2012; Zahir et al. 2012). As associate antecedent of AgNPs, leaf extracts of Manilkara zapota (L.) were applied to housefly (Kamaraj et al. 2012).
dsRNA
Vatanparast and Kim (2017) have shown that some insect pests will be controlled by RNA interference (RNAi) of a particular target cistron chymotrypsins (SeCHYs) to survive, the S. exigua, arthropod genus exigua, depends on construct insecticidal dsRNA in massive amounts since ribonucleinase activity within the midgut lumen reduces RNA interference (RNAi) activities. It’s counselled to focus on the young larvae that possess the very best levels of RNAi potency as a result of they possess a lot of RNase accelerator activity, giving them a lot of insecticidal activity against S. exigua.
The researchers have shown that even RNAi-mediated cistron silencing will be an alternate methodology of dominant pests of insects, as a result of it directly knocks down the target cistron through the oral administration of poly (diethylene glycol) or endocytosis (Katoch et al. 2013). To boot, with the event of biotechnological tools, more target genes are explored for insect tormenter management. It’s necessary to know the technique on every level. However, in order that forthcoming obstacles will be discovered. Insect pests area unit currently controlled victimization of double-stranded RNA (dsRNA) created by artificial means that diet plays an important role in preventing a species of Coleoptera is that the western corn rootworm, Diabrotica virgifera virgifera LeConte. Insects of the lepidopteran and coleopteran genera were the most raisers of insecticidal proteins created by true bacteria B. thuringiensis before the invention of engineering science. Within the current state of affairs of insect resistance emergence, thus, the event of various modes of biotechnological tools can be helpful (Baum et al. 2007). MicroRNAs (miRNAs) and short meddling RNAs (siRNAs) area unit RNAi biogenesis categories in insects (Burand and Hunter 2013). By physical science and physical mechanisms, nanostructured corundum (NSA) bestowed to kill insect pests. Electrons are generated by oxidizing metals and creating particle-based electrical charges. Outwardly connected insects turn out turboelectric assessments. United States intelligence agency involves two steps: first, charged insect particles area unit electrically sure to charge United States intelligence agency particles. A second reason is that United States intelligence agency particles, because of their powerful sorbtive properties, take away the insect’s cuticle, that causes dehydration, that ultimately kills the insect. Because of static discharge, particles adhere to insect cuticle surfaces disrupting water balance, and their effectiveness declines as close humidness will increase. As results of its intrinsic charge, tiny particle size, and huge specific area, the United States intelligence agency incorporates a high insecticidal effectiveness.
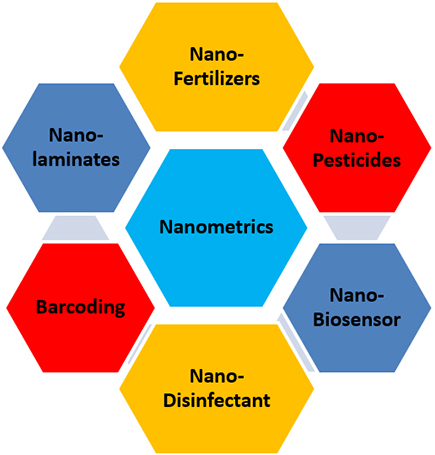
Figure 4
Components of nanotechnology
Nanobiopesticides: challenges and futuristic approaches
There may be an encouragement for nanopesticides in agriculture. Still, people's seats affect unchangeable wellness difficulties to the neurotic way if they intersect the blood-brain, blood-placental, and blood-retina barriers. By manipulating the molecular configuration of textiles, nanotechnology can modify their inherent possessions and convey others with rebellious applications (Fig. 4). In appreciation to investigating the element configuration of the staple matter, nanopesticides are currently being scrutinized for their mechanical and biochemical attributes, such as intensity, electrical ammunition, and exterior qualities, which pretend significant intimidation to human fitness (Zielińska et al. 2020). Increasing resistance to a group of insect pests could cause the plant to become more aggressive as a weed. Rapeseed genetically engineered for insect resistance was shown to have a better reproductive chance than nontransgenic rapeseed in experiments imposing strong herbivorous insects’ selective pressure (Stewart et al. 1997). During its growth, weeds that can resist certain insect pests may become more aggressive. Genetically modified rapeseed showed a better reproductive potential under selective pressure from insect pests (Stewart et al. 1997) compared with nontransgenic rapeseed. Transgenic plants' effects on pollinators will trust heavily the investigation of the gene entangled and its declaration in plants that honeybees swallow (Malone and Pham-Delègue 2001). The environmental risk is associated with the fluidity of genetic material within and between species (Chevre et al. 1996; Darmency 1994). Nanotechnology applications can lead to more environmentally friendly biopesticides, more stable active ingredients, improved efficacy, broader adoption, easy to apply, can be targeted in delivery, significantly reduce pesticide load, and protect microbiota and contribute to biopesticide degradation (Deshpande 2019). Certain biological substances can be made more effective against pests, less toxic to humans and the environment, encapsulated in nanoparticle systems with nanotechnology, and reduced physical degradation (Medin-Pérez et al. 2019). Promoting sustainable and eco-friendly pest management strategies with synthetic pesticides is exciting in modern entomology, which sits as sundry jeopardies for human and environmental safety. An eco-friendly pest control route is possible by using “green” pesticides in conjunction with nanotechnology (Pavoni et al. 2019).
It is possible to use nanobiotechnology to manage insect pests in different ways, and it presents great potential for use in integrated pest management systems. Enhanced control of pests and disease vectors will be a massive benefit if this technology is used. Substituting protease inhibitors and Bt-δ-endotoxins for conventional insecticides could educate selection stress, slowed resistance development. There would be no need to continuously monitor pests since all plant parts would be covered with toxins. Furthermore, transgenic plants would protect plant parts that are difficult to treat with insecticides. Low cultivation costs. Drift and groundwater contamination are low. Crop plants would only be targeted by insecticidal activity. Animals and humans not targeted are safe. Biosynthesis of the toxins occurs on-site. Agricultural merchandise can be concocted, stored, packaged, and transported using nanotechnology at every stage in the process. Nanotechnology-based synthesis of potential or controlled-release pesticides has demonstrated infinite effectiveness over other pesticide performance practices. Biological compe-tency is achieved when the agrochemicals are released at a measured amount over a specified period to minimize losses or adverse effects (Shojaei et al. 2019). By combining nano-particles with other substances, new formulations can be created, such as pesticides, insecticides, insect repellents, pheromones, and fertilizers. The necessary offering arrangement ensures a maintained deliverance of the vital constituent at the scapegoat position over time. The addition of the pesticide efficacy time decreases the regularity of pesticide application. Reducing the chemical input to plants by improving bioefficacy. Limiting photo-degradation of light-sensitive compounds to reduce non-target toxicity. In the ecosystem, pesticides are released over time to protect biodiversity (Hayles et al. 2017; Yu et al. 2017).
Food and agriculture suffer numerous difficulties in the present world today. Nanotechnology is one potential and securing midpoints to attain a clarification. By manipulating a molecule's attributes, nanomanipulation can imagine complexes that augment the inherent utilization in agriculture (Lade et al. 2019). According to Joshi et al. (2019), nanotechnology in agriculture is principally utilized to depreciate the volume of blanket chemicals, diminish nutrient impairments throughout fertilization, and enhance crop yields through nutrient and pest management. Use of nanotechnology in nutrient mana-gement, nanocoating, nanoencapsulation, plant regulators, precision agriculture, pest and disease management, agronomy and environmental sensitivity, genetic manipulation of crops, application in food industries (Food Packaging & Food Processing and Safety), etc.
The literature has announced numerous new compounds that can be used as biopesticides (Table 1). Still, additional field investigation is required to conclude whether they are effective against specific pests in different cropping operations. Clitoria ternatea L. extract against H. armigera species (Mensah et al. 2014), B. thuringiensis var. tenebrionis strain Xd3 (Btt-Xd3) against Agelastica alni (L.) (Eski et al. 2017), alkaloid compound oxymatrine against Spodoptera litura (Fabricius), H. armigera and Aphis gossypii Glover (Rao and Kumari 2016), stilbenes isolated from grapevine extracts against S. littoralis (Pavela et al. 2017) and olive mill waste against various insect pests (El-Abbassi et al. 2017). Insignificant elements, nanoparticles occupy distinct chemical, optical, mechanical, and irresistible properties because of their large exterior stretches (Bhan et al. 2018). Efficient enforcement methods for water and fertilizer, tiny bionanocapsules, and nanosensors for vector and pest management can improve agricultural potency. There is no proxy for conventional strategies to pest management, and spraying indiscriminately with toxic chemicals has adverse effects on the environment, animals, and humans. Thus, nanotechnology presents an excellent alternative to harmful chemical pesticides in agriculture. Nanotechnology-enhanced crops protection tools based on intensification crop production and productivity, enhancement crop tolerance, improvement in crop growth, produced transgenic crop plants, resistant varieties, food processing improvement, soil augmentation, operation correction, stretch sensitivity, etc. (Shang et al. 2019).
Table 1
Nanoemulsion sources in different crop plants
CONCLUSION
Bionanotechnology is an unprecedented new engendering tool for looking at insects and is conceivably essential for agriculture’s property, eco-friendly development within the gift. The technology might facilitate in reducing the utilization of harmful chemicals for crop protection and at the same time cut back the pollution burden related to chemical usage. Bionanotechnology tools have spectacular consequences alone as they need consecutive importance and facilitate with tormenter supervision in improvement to decreasing the price of facilities and operation. Additionally, it fits most of the distances while not poignant the areas though insects follow a dynamic equilibrium. Transgenic crops area unit unlikely to cause health issues for humans. However we have a tendency to not exclude health risks since there’s not enough info regarding them. New proteins might act as allergens or toxins, altered host metabolisms might end in new allergens or toxins, and dietary deficiencies or health issues might result from reduced nutritional value. To ensure the long-term security of food and to mitigate insect pest-induced organic phenomenon stress, these various methods are important. It’s conjointly helpful for college students, scientists, NGOs, directors, and researchers in developing some ways to combat insect pests.
Appendices
REFERENCES
- Almohamad, R., F.J. Verheggen, and É. Haubruge. 2009. Searching and oviposition behavior of aphidophagous hoverflies (Diptera: Syrphidae): a review. Biotechnol. Agron. Soc. Environ. 13: 467-481.
- Barraclough, E.I., E.P.J. Burgess, B.A. Philip, M.W. Wohlers, and L.A. Malone. 2009. Tritrophic impacts of Bt-expressing transgenic pine on the parasitoid Meteorus pulchricornis (Hymenoptera: Braconidae) via its host Pseudocoremia suavis (Lepidoptera: Geometridae). Biol. Control 49: 192-199.
- Baum, J.A., T. Bogaert, W. Clinton, G.R. Heck, P. Feldmann, O. Ilagan, S. Johnson, G. Plaetinck, T. Munyikwa, M. Pleau, T. Vaughn, and J. Roberts. 2007. Control of coleopteran insect pests through RNA interference. Nat. Biotechnol. 25: 1322-1326.
- Baur, M.E., and D.J. Boethel. 2003. Effect of Bt-cotton expressing Cry1A(c) on the survival and fecundity of two hymenopteran parasitoids (Braconidae, Encyrtidae) in the laboratory. Biol. Control 26: 325-332. doi:10.1016/S1049-9644(02)00160-3
- Bell, H.A., E.C. Fitches, G.C. Marris, J. Bell, J.P. Edwards, J.A. Gatehouse, and A.M.R. Gatehouse. 2001. Transgenic GNA expressing potato plants augment the beneficial biocontrol of Lacanobia oleracea (Lepidoptera; Noctuidae) by the parasitoid Eulophus pennicornis (Hymenoptera; Eulophidae). Transgenic Res. 10: 35-42. doi:10.1023/A:1008923103515
- Benelli, G. 2018. Gold nanoparticles – against parasites and insect vectors. Acta Trop. 178: 73-80.
- Bernal, J.S., J.G. Griset, and P.O. Gillogly. 2002. Impacts of developing on Bt maize-intoxicated hosts on fitness parameters of a stem borer parasitoid. J. Entomol. Sci. 37: 27-40.
- Bhan, S., L. Mohan, and C.N. Srivastava. 2018. Nanopesticides: a recent novel ecofriendly approach in insect pest mana-gement. J. Entomol. Res. 42: 263-270.
- Bicheng, H., C. Yuan, Y. Meizhen, M. Klaus, A. Chunjuv, and S. Jie. 2013. Fluorescent nanoparticle delivered dsRNA toward genetic control of insect pests. Adv. Mater. 25: 4580-4584.
- Budenberg, W.J., W. Powell, and S.J. Clark. 1992. The influence of aphids and honeydew on the leaving rate of searching aphid parasitoids from wheat plants. Entomol. Exp. Appl. 63: 259-264.
- Buitenhuis, R., J.N. McNeil, G. Boivin, and J. Brodeur. 2004. The role of honeydew in host searching of aphid hyper-parasitoids. J. Chem. Ecol. 30: 273-285.
- Bunge, M., N. Araghipour, T. Mikoviny, J. Dunkl, R. Schnitzhofer, A. Hansel, F. Schinner, A. Wisthaler, R. Margesin, and T.D. Märk. 2008. Online monitoring of microbial volatile metabolites by proton transfer reactionmass spectrometry. Appl. Environ. Microbiol. 74: 2179-2186.
- Burand, J.P., and W.B. Hunter. 2013. RNAi: future in insect management. J. Invertebr. Pathol. 112: S68-S74.
- Chakravarthy, A.K., A. Bhattacharyya, P.R. Shashank, T.T. Epidi, B. Doddabasappa, and S.K. Mandal. 2012. DNA-tagged nano gold: a new tool for the control of the armyworm, Spodoptera litura Fab. (Lepidoptera: Noctuidae). Afr. J. Biotechnol. 11: 9295-9301.
- Chakroun, M., N. Banyuls, Y. Bel, B. Escriche, and J. Ferre. 2016. Bacterial Vegetative insecticidal protein (Vip) from entomopathogenic bacteria. Microbiol. Mol. Biol. Rev. 80: 329-350.
- Chandra, J.H., L.F.A.A. Raj, S.K.R. Namasivayam, and R.S.A. Bharani. 2013. Improved pesticidal activity of fungal metabolite from nomureae rileyi with chitosan nano-particles. Pages 387-390 in T. Sasipraba (ed.), International conference on advanced nanomaterials and emerging engineering technologies. Institute of Electrical and Electronics Engineers (IEEE), Piscataway, NJ, USA. doi:10.1109/ICANMEET.2013.6609326
- Chandrashekharaiah, M., S.B. Kandakoor, G.B. Gowda, V. Kamar, and A.K. Chakravarthy. 2015. Nanomaterials: a review of their action and application in pest management and evaluation of DNA-tagged particles. Pages 113-126 in A.K. Chakravarthy (ed.), New horizons in insect science: towards sustainable pest management. Springer, New Delhi, India.
- Chèvre, A.M., F. Eber, A. Baranger, M.C. Kerlan, P. Barret, G. Festoc, P. Vallée, and M. Renard. 1996. Interspecific gene flow as a component of risk assessment for transgenic Brassicas. Acta Hortic. 407: 69-179.
- Choi, M.-Y., B.D. Roitberg, A. Shani, D.A. Raworth, and G.-H. Lee. 2004. Olfactory response by the aphidophagous gall midge, Aphidoletes aphidimyza to honeydew from green peach aphid, Myzus persicae. Entomol. Exp. Appl. 111: 37-45.
- Christeller, J.T., E.P.J. Burgess, V. Mett, H.S. Gatehouse, N.P. Markwick, C. Murray, L.A. Malone, M.A. Wright, B.A. Philip, D. Watt, L.N. Gatehouse, G.L. Lövei, A.L. Shannon, M.M. Phung, L.M. Watson, and W.A. Laing. 2002. The expression of a mammalian proteinase inhibitor, bovine spleen trypsin inhibitor in tobacco and its effects on Helicoverpa armigera larvae. Transgenic Res. 11: 161-173.
- Corbin, D.R., R.J. Grebenok, T.E. Ohnmeiss, J.T. Greenplate, and J.P. Purcell. 2001. Expression and chloroplast targeting of cholesterol oxidase in transgenic tobacco plants. Plant Physiol. 126: 1116-1128.
- Darmency, H. 1994. The impact of hybrids between genetically modified crop plants and their related species: introgression and weediness. Mol. Ecol. 3: 37-40.
- Deshpande, M.V. 2019. Nanobiopesticide perspectives for protection and nutrition of plants. Pages 47-68 in O. Koul (ed.), Nano-biopesticides today and future perspectives. Academic Press, London, United Kingdom.
- Devi, S., and S.S. Kanwar. 2017. Cholesterol oxidase: source, properties and applications. Insights Enzyme Res. 1: 1-12. doi:10.21767/2573-4466.100005
- Du, Y., G.M. Poppy, W. Powell, and L.J. Wadhams. 1997. Chemically mediated associative learning in the host foraging behavior of the aphid parasitoid Aphidius ervi (Hymenoptera: Braconidae). J. Insect Behav. 10: 509-522. doi.org/10.1007/BF02765374
- Duan, X., X. Li, Q. Xue, M. Abo-EI-Saad, D. Xu, and R. Wu. 1996. Transgenic rice plants harboring an introduced potato proteinase inhibitor II gene are insect resistant. Nat. Biotechnol. 14: 494-498.
- El-Abbassi, A., N. Saadaoui, H. Kiai, J. Raiti, and A. Hafidi. 2017. Potential applications of olive mill wastewater as biopesticide for crops protection. Sci. Total Environ. 576: 10-21.
- Eleftherianos, I., J. Marokhazi, P.J. Millichap, A.J. Hodgkinson, A. Sriboonlert, R.H. ffrench-Constant, and S.E. Reynolds. 2006. Prior infection of Manduca sexta with non-pathogenic Escherichia coli elicits immunity to pathogenic Photorhabdus luminescens: roles of immune-related proteins shown by RNA interference. Insect Biochem. Mol. Biol. 36: 517-525.
- Elek, N., R. Hoffman, U. Raviv, R. Resh, I. Ishaaya, and S. Magdassi. 2010. Novaluron nanoparticles: formation and potential use in controlling agricultural insect pests. Colloids Surf. A-Physicochem. Eng. Asp. 372: 66-72.
- English, C., and K. Schreiber. 2020. Where are GMO crops grown? GLP infographics document the global growth of agricultural biotechnology innovation. Available online [https://www.printfriendly.com/p/g/rG2CcH].
- Epsky, N.D., R.R. Heath, B.D. Dueben, C.R. Lauzon, A.T. Proveaux, and G.B. MacCollom. 1998. Attraction of 3methyl1butanol and ammonia identified from Enterobacter agglomerans to Anastrepha suspensa. J. Chem. Ecol. 24: 1867-1880.
- Eski, A., İ. Demir, K. Sezen, and Z. Demirbağ. 2017. New bio-pesticide from a local Bacillus thuringiensis var. tenebrionis (Xd3) against alder leaf beetle (Coleoptera: Chrysomelidae). World J. Microbiol. Biotechnol. 33: 95.
- Felton, G.W., J.L. Bi, C.B. Summers, A.J. Mueller, and S.S. Duffey. 1994. Potential role of lipoxygenases in defense against insect herbivory. J. Chem. Ecol. 20: 651-666.
- Feng, B.-H., and L.-F. Peng. 2012. Synthesis and characterization of carboxymethyl chitosan carrying ricinoleic functions as an emulsifier for azadirachtin. Carbohydr. Polym. 88: 576-582.
- Flores-Céspedes, F., C.I. Figueredo-Flores, I. Daza-Fernández, F. Vidal-Peña, M. Villafranca-Sánchez, and M. Fernández-Pérez, M. 2012. Preparation and characterization of imidacloprid lignin-polyethylene glycol matrices coated with ethylcellulose. J. Agric. Food Chem. 60: 1042-1051.
- Franco, O.L., D.J. Rigden, F.R. Melo, and M.F. Grossi-de-Sá. 2002. Plant α-amylase inhibitors and their interaction with insect α-amylases: structure, function and potential for crop protection. Eur. J. Biochem. 269: 397-412.
- Frandsen, M.V., M.S. Pedersen, M. Zellweger, S. Gouin, S.D. Roorda, and T.Q.C. Phan. 2010. Insecticidal polymer matrix comprising HDPE and LDPE. Publication no WO2010015256A2. Available online [https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2010015256].
- Gaines, J.C. 1957. Cotton insects and their control in the United Sates. Annu. Rev. Entomol. 2: 319-338.
- Gatehouse, A.M.R., G.M. Davison, C.A. Newell, A. Merryweather, W.D.O. Hamilton, E.P.J. Burgess, R.J.C. Gilbert, and J.A. Gatehouse. 1997. Transgenic potato plants with enhanced resistance to the tomato moth, Lacanobia oleracea: growth room trials. Mol. Breed. 3: 49-63.
- Geng, J.H., Z.R. Shen, K. Song, and L. Zheng. 2006. Effect of pollen of regular cotton and transgenic Bt + CpTi cotton on the survival and reproduction of the parasitoid wasp Trichogramma chilonis (Hymenoptera: Trichogrammatidae) in the laboratory. Environ. Entomol. 35: 1661-1668.
- Goldshtein, R., I. Jaffe, and B. Tulbovitz. 2005. Hydrophilic dispersions of nanoparticles of inclusion complexes of amorphous compounds. Publication no WO2006106519. Available online [https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2006106519].
- Gopal, M., R. Kumar, and A. Goswami. 2012. Nano-pesticides - a recent approach for pest control. J. Plant Prot. Sci. 4: 1-7.
- Government of India. 2020. Cultivation of genetically modified crops. Press Information Bureau. Available online [https://pib.gov.in/PressReleasePage.aspx?PRID=1605056].
- Harada, H., H. Oyaizu, and H. Ishikawa. 1996. A consideration about the origin of aphid intracellular symbiont in connection with gut bacterial flora. J. Gen. Appl. Microbiol. 42: 17-26.
- Hayles, J., L. Johnson, C. Worthley, and D. Losic. 2017. Nano-pesticides: a review of current research and perspectives. Pages 193-225 in A.M. Grumezescu (ed.), New pesticides and soil sensors. Academic Press, London, United Kingdom.
- Haynes, S., A.C. Darby, T.J. Daniell, G. Webster, F.J.F. Van Veen, H.C.J. Godfray, J.I. Prosser, and A.E. Douglas. 2003. Diversity of bacteria associated with natural aphid populations. Appl. Environ. Microbiol. 69: 7216-7223.
- Hellmann, C., A. Greiner, and J.H. Wendorff. 2011. Design of pheromone releasing nanofibers for plant protection. Polym. Adv. Technol. 22: 407-413.
- Hogervorst, P.A.M., F.L. Wäckers, and J. Romeis. 2007. Effects of honeydew sugar composition on the longevity of Aphidius ervi. Entomol. Exp. Appl. 122: 223-232.
- Hussain, A., J.M.S. Forrest, and A.F.G. Dixon. 1974. Sugar, organic acid, phenolic acid and plant growth regulator content of extracts of honeydew of the aphid Myzus persicae and of its host plant, Raphanus sativus. Ann. Appl. Biol. 78: 65-73.
- ISAAA. 2018. Brief 54: Global status of commercialized biotech/GM crops in 2018: Biotech crops continue to help meet the challenges of increased population and climate change. Ithaca, NY, USA, 100 pp. Available online [https://www.isaaa.org/resources/publications/briefs/54/download/isaaa-brief-54-2018.pdf]
- Johnson, M.T., and F. Gould. 1992. Interaction of genetically engineered host plant resistance and natural enemies of Heliothis virescens (Lepidoptera: Noctuidae) in tobacco. Environ. Entomol. 21: 586-597.
- Joshi, H., Somdutt, P. Choudhary, and S.L. Mundra. 2019. Future prospects of nanotechnology in agriculture. Int. J. Chem. Stud. 7: 957-963.
- Kai, M., M. Haustein, F. Molina, A. Petri, B. Scholz, and B. Piechulla. 2009. Bacterial volatiles and their action potential. Appl. Microbiol. Biotechnol. 81: 1001-1012.
- Kamaraj, C., G. Rajakumar, A.A. Rahuman, K. Velayutham, A. Bagavan, A.A. Zahir, and G. Elango. 2012. Feeding deterrent activity of synthesized silver nanoparticles using Manilkara zapota leaf extract against the house fly, Musca domestica (Diptera: Muscidae). Parasitol. Res. 111: 2439-2448.
- Katoch, R., A. Sethi, N. Thakur, and L.L. Murdock. 2013. RNAi for insect control: current perspective and future challenges. Appl. Biochem. Biotechnol. 171: 847-873.
- Kulkarni, A.R., K.S. Soppimath, T.M. Aminabhavi, A.M. Dave, and M.H. Mehta. 1999. Application of sodium alginate beads crosslinked with glutaraldehyde for controlled release of pesticide. Carbohydr. Polym. 24: 285-286.
- Lade, B.D., D.P. Gogle, D.B. Lade, G.M. Moon, S.B. Nandeshwar, and S.D. Kumbhare. 2019. Nanobiopesticide formulations: application strategies today and future perspectives. Pages 179-206 in O. Koul (ed.), Nano-biopesticides today and future perspectives. Academic Press, London, United Kingdom.
- Lao, S.-B., Z.-X. Zhang, H.-H. Xu, and G.-B. Jiang. 2010. Novel amphiphilic chitosan derivatives: synthesis, characterization and micellar solubilization of rotenone. Carbohydr. Polym. 82: 1136-1142.
- Lau, Y.S., S. Sulaiman, and H. Othman. 2011. The effectiveness of trypsin modulating oostatic factor (TMOF) and combination of TMOF with Bacillus thuringiensis against Aedes aegypti larvae in the laboratory. Iran. J. Arthropod Borne Dis. 5: 13-19.
- Lee, J.C., G.E. Heimpel, and G.L. Leibee. 2004. Comparing floral nectar and aphid honeydew diets on the longevity and nutrient levels of a parasitoid wasp. Entomol. Exp. Appl. 111: 189-199.
- Leroy, P.D., A. Sabri, S. Heuskin, P. Thonart, G. Lognay, F.J. Verheggen, F. Francis, Y. Brostaux, G.W. Felton, and E. Haubruge. 2011. Microorganisms from aphid honeydew attract and enhance the efficacy of natural enemies. Nat. Commun. 2: 348.
- Leroy, P.D., B. Wathelet, A. Sabri, F. Francis, F.J. Verheggen, Q. Capella, P. Thonart, and E. Haubruge. 2011. Aphid-host plant interactions: does aphid honeydew exactly reflect the host plant amino acid composition? Arthropod Plant Interact. 5: 193-199.
- Li, M., Q. Huang, and Y. Wu. 2011. A novel chitosan-poly(lactide) copolymer and its submicron particles as imidacloprid carriers. Pest Manag. Sci. 67: 831-836.
- Liu, X., Q. Zhang, J.-Z. Zhao, Q. Cai, H. Xu, and J. Li. 2005. Effects of the Cry1Ac toxin of Bacillus thuringiensis on Microplitis mediator, a parasitoid of the cotton bollworm, Helicoverpa armigera. Entomol. Exp. Appl. 114: 205-213.
- Macrae, T.C., M.E. Baur, D.J. Boethel, B.J. Fitzpatrick, A.-G. Gao, J.C. Gamundi, L.A. Harrison, V.T. Kabuye, R.M. Mcpherson, J.A. Miklos, M.S. Paradise, A.S. Toedebusch, and A. Viegas. 2005. Laboratory and field evaluations of transgenic soybean exhibiting high-dose expression of a synthetic Bacillus thuringiensiscry1A gene for control of Lepidoptera. J. Econ. Entomol. 98: 577-587.
- Malone, L.A., and M.-H. Pham-Delègue. 2001. Effects of transgene products on honey bees (Apis mellifera) and bumblebees (Bombus sp.). Apidologie 32: 287-304.
- Mao, Y.-B., W.-J. Cai, J.-W. Wang, G.-J. Hong, X.-Y. Tao, L.-J. Wang, Y.-P. Huang, and X.-Y. Chen. 2007. Silencing a cotton bollworm P450 monooxygenase gene by plant-mediated RNAi impairs larval tolerance of gossypol. Nat. Biotechnol. 25: 1307-1313.
- Medina-Pérez, G., F. Fernández-Luqueño, R.G. Campos-Montiel, K.B. Sánchez-López, L.N. Afanador-Barajas, and L. Prince. 2019. Nanotechnology in crop protection: status and future trends. Pages 17-45 in O. Koul (ed.), Nano-biopesticides today and future perspectives. Academic Press, London, United Kingdom.
- Mensah, R., C. Moore, N. Watts, M.A. Deseo, P.G. Glennie, and A. Pitt. 2014. Discovery and development of a new semiochemical biopesticide for cotton pest management: assessment of extract effects on the cotton pest Helicoverpa spp. Entomol. Exp. Appl. 152: 1-15.
- Mittler, T.E. 1958. Studies on the feeding and nutrition of Tuberolachnus salignus (Gmelin) (Homoptera, Aphididae): II. The nitrogen and sugar composition of ingested phloem sap and excreted honeydew. J. Exp. Biol. 35: 74-84.
- Naranjo, S.E. 2005. Long-term assessment of the effects of transgenic Bt cotton on the abundance of nontarget arthropod natural enemies. Environ. Entomol. 34: 1193-1210. doi:10.1093/ee/34.5.1193
- Oerke, E.-C. 2006. Crop losses to pests. J. Agric. Sci. 144: 31-43.
- Panáček, A., M. Kolář, R. Večeřová, R. Prucek, J. Soukupová, V. Kryštof, P. Hamal, R. Zbořil, L and L. Kvítek. 2009. Antifungal activity of silver nanoparticles against Candida spp. Biomaterials 30: 6333-6340.
- Pavela, R., P. Waffo-Teguo, B. Biais, T. Richard, and J.-M. Mérillon. 2017. Vitis vinifera canes, a source of stilbenoids against Spodoptera littoralis larvae. J. Pest Sci. 90: 961-970.
- Pavoni, L., G. Benelli, F. Maggi, and G. Bonacucina. 2019. Green nanoemulsion interventions for biopesticide formulations. Pages 133-160 in O. Koul (ed.), Nano-biopesticides today and future perspectives. Academic Press, London, United Kingdom.
- Pilcher, C.D., M.E. Rice, and J.J. Obrycki. 2005. Impact of transgenic Bacillus thuringiensis corn and crop phenology on five nontarget arthropods. Environ. Entomol. 34: 1302-1316.
- Prütz, G., and K. Dettner. 2004. Effect of Bt corn leaf suspension on food consumption by Chilo partellus and life history parameters of its parasitoid Cotesia flavipes under laboratory conditions. Entomol. Exp. Appl. 111: 179-187.
- Rao, P.V.M., and A. Kumari. 2016. Effect of oxymatrine 0.5% EC on predators and parasites of important pests on certain vegetable crops cultivated in Ranga Reddy District (Telangana). Pestology 40: 15-18.
- Robacker, D.C., and C.R. Lauzon. 2002. Purine metabolizing capability of Enterobacter agglomerans affects volatiles production and attractiveness to Mexican fruit fly. J. Chem. Ecol. 28: 1549-1563. doi:10.1023/A:1019920328062
- Robacker, D.C., A.J. Martinez, J.A. Garcia, and R.J. Bartelt. 1998. Volatiles attractive to the Mexican fruit fly (Diptera: Tephritidae) from eleven bacteria taxa. Fla. Entomol. 81: 497-508.
- Sanders, C.J., J.K. Pell, G.M. Poppy, A. Raybould, M. Garcia-Alonso, and T.H. Schuler. 2007. Host-plant mediated effects of transgenic maize on the insect parasitoid Campoletis sonorensis (Hymenoptera: Ichneumonidae). Biol. Control 40: 362-369.
- Santhoshkumar, T., A.A. Rahuman, G. Rajakumar, S.Marimuthu, A. Bagavan, C. Jayaseelan, A.A. Zahir, G.Elango, and C. Kamaraj. 2011. Synthesis of silver nano-particles using Nelumbo nucifera leaf extract and its larvicidal activity against malaria and filariasis vectors. Parasitol. Res. 108: 693-702.
- Sanyal, I., A.K. Singh, M. Kaushik, and D.V. Amla. 2005. Agrobacterium-mediated transformation of chickpea (Cicer arietinum L.) with Bacillus thuringiensis cry1Ac gene for resistance against pod borer insect Helicoverpa armigera. Plant Sci. 168: 1135-1146.
- Savary, S., P.S. Teng, L. Willocquet, and F.W. Nutter, Jr. 2006. Quantification and modeling of crop losses: a review of purposes. Annu. Rev. Phytopathol. 44: 89-112.
- Schellenberger, U., J. Oral, B.A. Rosen, J.-Z. Wei, G. Zhu, W. Xie, M.J. McDonald, D.C. Cerf, S.H. Diehn, V.C. Crane, G.A. Sandahl, J.-Z. Zhao, T.M. Nowatzki, A. Sethi, L. Liu, Z. Pan, Y. Wang, A.L. Lu, G. Wu, and L. Liu.2016. A selective insecticidal protein from Pseudomonas for controlling corn rootworms. Science 354: 634-637.
- Scholz, D., and H.-M. Poehling. 2000. Oviposition site selection of Episyrphus balteatus. Entomol. Exp. Appl. 94: 149-158.
- Schuler, T.H., I. Denholm, S.J. Clark, C.N. Stewart, and G.M. Poppy. 2004. Effects of Bt plants on the development and survival of the parasitoid Cotesia plutellae (Hymenoptera: Braconidae) in susceptible and Bt-resistant larvae of the diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae). J. Insect Physiol. 50: 435-443.
- Schulz, S., and J.S. Dickschat. 2007. Bacterial volatiles: the smell of small organisms. Nat. Prod. Rep. 24: 814-842.
- Sellami, S., K. Jamoussi, E. Dabbeche, and S. Jaoua. 2011. Increase of the Bacillus thuringiensis secreted toxicity against Lepidopteron larvae by homologous expression of the vip3LB gene during sporulation stage. Curr. Microbiol. 63: 289.
- Shang, Y., K. Hasan, G.J. Ahammed, M. Li, H. Yin, and J. Zhou. 2019. Applications of nanotechnology in plant growth and crop protection: a review. Molecules 24: 2558.
- Shankar, S.S., A. Rai, A. Ahmad, and M. Sastry. 2004. Rapid synthesis of Au, Ag, and bimetallic Au core-Ag shell nanoparticles using Neem (Azadirachta indica) leaf broth. J. Colloid Interface Sci. 275: 496-502.
- Sharma, A., K. Sood, J. Kaur, and M. Khatri. 2019. Agro-chemical loaded biocompatible chitosan nanoparticles for insect pest management. Biocatal. Agric. Biotechnol. 18: 101079.
- Sharma, H.C., and M.V. Rao. (eds.). 1995. Pests and pest management in India: the changing scenario. Plant Protection Association of India, Rajendranagar, Andhra Pradesh, India. 312 pp.
- Sharma, K. 2015. Protease inhibitors in crop protection from insects. Int. J. Curr. Res. Acad. Rev. 3: 55-70.
- Shojaei, T.R., M.A.M. Salleh, M. Tabatabaei, H. Mobli, M. Aghbashlo, S.A. Rashid, and T. Tan. 2019. Applications of nanotechnology and carbon nanoparticles in agriculture. Pages 247-277 in S.A. Rashid, R.N.I. Raja Othman, and M.Z. Hussein (eds.), Synthesis, technology and applications of carbon nanomaterials. Elsevier, Amsterdam, Netherlands.
- Shukle, R.H., and L.L. Murdock. 1983. Lipoxygenase trypsin inhibitor, and lectin from soybeans: effects on larval growth of Manduca sexta (Lepidoptera: Sphingidae). Environ. Entomol. 12: 787-791.
- Sparks, T.C., J.E. Dripps, G.B. Watson, and D. Paroonagian. 2012. Resistance and cross-resistance to the spinosyns - a review and analysis. Pestic. Biochem. Physiol. 102: 1-10.
- Stewart, C.N., J.N. All, P.L. Raymer, and S. Ramachandran. 1997. Increased fitness of transgenic insecticidal rapeseed under insect selection pressure. Mol. Ecol. 6: 773-779.
- Swaminathan, M.S. 2000. Genetic engineering and food security: ecological and livelihood issues. Pages 37-44 in G.J. Persley, and M.M. Lantin (eds.), Agricultural bio-technology and the rural poor. Consultative Group on International Agricultural Research, Washington, DC, USA.
- Tamhane, V.A., A.P. Giri, M.N. Sainani, and V.S. Gupta. 2007. Diverse forms of Pin-II family proteinase inhibitors from Capsicum annuum adversely affect the growth and development of Helicoverpa armigera. Gene 403: 29-38.
- Tohidfar, M., B. Ghareyazie, M. Mosavi, S. Yazdani, and R. Golabchian. 2008. Agrobacterium-mediated transformation of cotton (Gossypium hirsutum) using a synthetic cry1Ab gene for enhanced resistance against Heliothis armigera. Iran. J. Biotechnol. 6: 164-173.
- Tortiglione, C., P. Fanti, F. Pennacchio, C. Malva, M. Breuer, A. De Loof, L.M. Monti, E. Tremblay, and R. Rao. 2002. The expression in tobacco plants of Aedes aegypti Trypsin Modulating Oostatic Factor (Aea-TMOF) alters growth and development of the tobacco budworm, Heliothis virescens. Mol. Breed. 9: 159-169.
- Valencia-Jiménez, A., J.W. Arboleda, A. López Ávila, and M.F. Grossi-de-Sá. 2008. Digestive α-amylases from Tecia solanivora larvae (Lepidoptera: Gelechiidae): response to pH, temperature and plant amylase inhibitors. Bull. Entomol. Res. 98: 575-579.
- Vatanparast, M., and Y. Kim. 2017. Optimization of recombinant bacteria expressing dsRNA to enhance insecticidal activity against a lepidopteran insect, Spodoptera exigua. PLoS One 12: e0183054.
- Vendan, S.E., K. Baskar, M.G. Paulraj, and S. Ignacimuthu. 2009. Antifeedant and larvicidal effects of Hydnocarpus alpine Wt. (Flacourtiaceae) extracts against the larvae of Helicoverpa armigera Hub. (Lepidoptera: Noctuidae). Pages 210-216 in S. Ignacimuthu, and B.V. David (eds.), Ecofriendly insect pest management. Elite Publishing House, New Delhi, India.
- Verheggen, F.J., L. Arnaud, S. Bartram, M. Gohy, and E. Haubruge. 2008. Aphid and plant volatiles induce oviposition in an aphidophagous hoverfly. J. Chem. Ecol. 34: 301-307.
- Wäckers, F.L. 2000. Do oligosaccharides reduce the suitability of honeydew for predators and parasitoids? A further facet to the function of insectsynthesized honeydew sugars. Oikos 90: 197-201.
- Wright, J.E. 1997. Formulation for insect sex pheromone dispersion. Patent number US 5670145 A 19970923.
- Yu, M., J. Yao, J. Liang, Z. Zeng, B. Cui, X. Zhao, C. Sun, Y. Wang, G. Liu, and H. Cui. 2017. Development of functionalized abamectin poly(lactic acid) nanoparticles with regulatable adhesion to enhance foliar retention. RSC Adv. 7: 11271-11280.
- Zahir, A.A., A. Bagavan, C. Kamaraj, G. Elango, and A.A. Rahuman. 2012. Efficacy of plant-mediated synthesized silver nanoparticles against Sitophilus oryzae. J. Biopestic. 5: 95-102.
- Zahir, A.A., A.A. Rahuman. 2012. Evaluation of different extracts and synthesised silver nanoparticles from leaves of Euphorbia prostrata against Haemaphysalis bispinosa and Hippobosca maculata. Vet. Parasitol. 187: 511-520.
- Zielińska, A., B. Costa, M.V. Ferreira, D. Miguéis, J.M.S. Louros, A. Durazzo, M. Lucarini, P. Eder, M.V. Chaud, M. Morsink, N. Willemen, P. Severino, A. Santini, and E.B. Souto. 2020. Nanotoxicology and nanosafety: safety-by-design and testing at a glance. Int. J. Environ. Res. Public Health 17: 4657.
List of figures
Figure 1
Interlinking of domains
Figure 2
Year-wise area under cotton, Bt cotton, production and yield of cotton during 2002-03 to 2019-20
Figure 3
Global area of biotech crops, 1996 to 2018
Figure 4
Components of nanotechnology
List of tables
Table 1
Nanoemulsion sources in different crop plants