Abstracts
Abstract
Root-knot nematode is a major constraint to tomato cultivation in open and protected structures. Resistance sources need to be continuously identified for combating pathogens affecting the yield. In the present studies, forty-seven genotypes of tomato were evaluated phenotypically along with their genotypic characterization. On the basis of their phenotypic reaction, the genotypes were grouped into four categories viz.: resistant, moderately resistant, susceptible and highly susceptible. Of these genotypes, only five were found to be resistant while forty-two were rated from moderately resistant to highly susceptible. Multiplication of Meloidogyne incognita was greatly reduced (Rf < 1) in resistant genotypes as compared to susceptible genotypes. Root galling index was also very low in resistant genotypes. Using molecular markers, the presence of the Mi-1.2 resistance gene was also confirmed in five resistant genotypes (L-0272, NR-14, L-097, L-0275 and PNR-7). These resistant sources could become a source of germplasm in breeding programs for the development of resistant cultivars.
Keywords:
- tomato,
- screening,
- Mi gene,
- resistance,
- Meloidogyne incognita
Résumé
Le nématode à galles est une contrainte majeure à la culture de la tomate dans des structures ouvertes et protégées. Les sources de résistance doivent être identifiées en permanence pour lutter contre les agents pathogènes affectant le rendement. Jusqu’à présent, quarante-sept génotypes de tomate ont été évalués phénotypiquement, de même que leur caractérisation génotypique. Selon leur réaction phénotypique, les génotypes ont été regroupés en quatre catégories : résistant, modérément résistant, sensible et très sensible. Parmi ces génotypes, seuls cinq se sont révélés résistants tandis que quarante-deux ont été classés de modérément résistants à très sensibles. La multiplication de Meloidogyne incognita était fortement réduite (Rf < 1) dans les génotypes résistants par rapport aux génotypes sensibles. L'indice de galles racinaires était également très faible dans les génotypes résistants. À l'aide de marqueurs moléculaires, la présence du gène de résistance Mi-1.2 a également été confirmée dans cinq génotypes résistants (L-0272, NR-14, L-097, L-0275 et PNR-7). Ces sources résistantes pourraient devenir une source de matériel génétique dans les programmes de sélection pour le dévelop-pement de cultivars résistants.
Mots-clés :
- tomate,
- criblage,
- gène Mi,
- résistance,
- Meloidogyne incognita
Article body
INTRODUCTION
Tomato (Solanum lycopersicum L.) is one of the most popular vegetable crops grown worldwide owing to its high-diversified use and nutritive value. It is grown in open as well as in protected cultivation. In India, tomato is cultivated on 781 000 ha with a production of 19 007 000 metric tons in 2018-2019 (Indiastat 2019). In Punjab, tomato is grown on 10 170 ha with 252 630 tons of production during year 2019 (Anonymous 2019). Tomato cultivation under poly house is increasing in Punjab due to offseason cultivation, increased yield and more economical benefits. A number of biotic and abiotic stresses impose constraints to the production of this crop. Among these, root-knot nematodes (RKN, Meloidogyne spp.) have become a cause of concern for production of tomato in open as well as poly house cultivation and are reported to cause yield losses ranging from 25 to 100% worldwide (Seid et al. 2015). The losses under protected cultivation of tomato are even more due to continuous survival, increased number of generations and availability of susceptible hosts (Shurtleff and Averre 2000). The conducive temperature and persistent moisture prevalent in poly houses further add up to their increased infestation and hence strategies need to be developed for management of these pathogens.
Tomato being a directly consumable crop, application of nematicides not only affects environment but poses health concerns also. In view of this, there is urgent need for exploring alternative options for nematode management. The use of host plant resistance is a viable, less expensive and environmentally safe strategy in integrated nematode management approaches. The use of tomato genotypes containing the Mi gene or incorporating the gene in new cultivars is a useful approach to avert the losses caused by RKN species (Bozbuqa et al. 2020). Goggin et al. (2001) observed that resistance given by the Mi-1.2 gene in tomato is highly specialized. The Mi gene imparts resistance to the plant as early as two weeks after germination (El-Sappah et al. 2019). This gene confers resistance to three of the most damaging species of RKN viz.: M. incognita, M. arenaria and M. javanica (Milligan et al. 1998). The present study was therefore conducted to identify sources of resistance in tomato against M. incognita along with molecular confirmation of the presence of the Mi-1.2 locus in resistant genotypes.
MATERIALS AND METHODS
Pure culture of M. incognita was maintained in pot house by single egg mass technique (Zakaria et al. 2013). Galled roots of tomato plant uprooted from the sick plot of M. incognita maintained in the Department of Plant Pathology, Ludhiana, were gently washed with water to remove the adhering soil particles. A single egg mass of M. incognita was picked by hand with a fine forceps and was surface-sterilized with 0.5% aqueous solution of sodium hypochlorite for 2 min followed by washing with distilled water. Then the egg mass was transferred to a small coarse sieve lined with tissue paper to cover the bottom of the sieve that was within a Petri plate containing sufficient amount of water. The Petri plate was incubated at room temperature (27 ± 5 °C) for 24 h (den Ouden 1958). Tomato seedlings grown in autoclaved soil were inoculated with the progeny of the single egg mass in order to get regular supply of the inoculum for the experiment. The extracted nematode was identified as M. incognita according to the perineal patterns of the mature female. It illustrated the presence of a high, squarish dorsal arch, which contains a distinct whorl in the tail terminal area. The striae are smooth to wavy. Distinct lateral lines are absent, but breaks and forks in striae are obvious. Forty-seven genotypes of tomato (Table 1) available with the Department of Vegetable Science, Punjab Agricultural University, Ludhiana, were screened against M. incognita for two years (2016 and 2017). Experiment was carried out on a loamy sand soil (75.5% sand; 19.3% silt; 5.2% clay; pH 7.6; EC: 0.24 dS m-1; organic matter: 0.672%). During the year 2016, the temperature, relative humidity and sunshine hours during October month were recorded to be 25.9 °C, 63.1% and 6.2 days respectively and in 2017, the temperature, relative humidity and sunshine hours during October month were recorded to be 27 °C, 73% and 8.42 days respectively. In order to accommodate 47 genotypes in three replications, total 141 pots (8-inch diameter) were filled with autoclaved soil along with tomato cultivar-Punjab Ratta which served as susceptible check. Five seeds of each genotype were sown in pots (8-inch diameter) containing 1 kg of autoclaved soil and each genotype was replicated thrice during both the years along with tomato cultivar-Punjab Ratta which served as susceptible check. At three-leaf stage, these were inoculated with M. incognita second stage juveniles extracted from the pure culture at a rate of 1 nematode (J2) per gram of soil.
Sixty days after sowing, growth parameters, root galling index (RGI), soil nematode population and reproduction factor were recorded. For growth parameters observations were recorded on plant height (cm), fresh shoot and root weight (g). Shoot and root length of individual plant was measured using cm scale. Fresh shoot and root weight was taken on a digital balance immediately after uprooting the plants from the pots. For fresh root weight, roots were cut from the plant with a pair of scissors and gently washed in running water and then excess water was dried by placing it on a blotting paper. For M. incognita population estimation in soil, a 250 g soil sample was taken from each pot and washed as per modified Cobb’s sieving and decanting method (Cobb 1918). The presence of M. incognita in roots was estimated on the basis of the RGI, a 0–5 scale given by Taylor and Sasser (1978). In this scale; 0 = no galls; 1 = 1–2; 2 = 3–10; 3 = 11–30; 4 = 31–100; and 5 = more than 100 galls. On the basis of RGI, the plants were rated for resistance where 0 = immune (I); 0.1–1.0 = resistant (R); 1.1–2.0 = moderately resistant; 2.1–3.0 = susceptible and 3.1–5.0 = highly susceptible (Begum et al. 2014). To estimate multiplication of the nematode in different genotypes, the reproduction factor (Rf) was calculated as the ratio of final nematode population to initial nematode population (Rf = Pf/Pi), where Pf = final population; Pi = initial population. Rf > 1 denotes reproduction of M. incognita while Rf < 1 implied no reproduction.
Statistical analyses were carried out with the SPSS software. The data were subjected to analysis of variance and differences among means were compared by Dunnett test (P < 0.05).
For genotypic characterization, genomic DNA from leaves of five resistant tomato genotypes (L-0272, NR-14, L-097, L-0275 and PNR-7) and three susceptible genotypes (Punjab Upma, Punjab Ratta and EC-535580) was extracted using CTAB method (Lodhi et al. 1994). The DNA samples were subjected to PCR amplification for the presence of the Mi gene, which is linked to the RKN resistance locus Mi-1.2 using SCAR marker Mi23 (F5’- TGG AAA AAT GTT GAA TTT CTT TTG-3’, and R5’- GCA TAC TAT ATG GCT TGT TTA CCC-3’) (Seah et al. 2007). For PCR, 25 μl reaction mix was prepared which comprises of 2 μl of template DNA (50 ng μl-1), 0.25 μl (500 unit) of Taq DNA polymerase (Promega, USA), 1.8 μl (25 mM) MgCl2, 0.8 μl (10 mM) dNTPs, 0.5 μl (12.5 μM) of each forward and reverse primers in 5 μl of reaction buffer (5×) (Green Go taq, Promega) and nuclease free water to make the final volume to 25 μl. The amplification was carried out using an Eppendorf PCR system AG 22331 thermal cycler (Eppendorf, Germany) and the program included one initial cycle of denaturation at 94 °C for 3 min, 35 cycles of denaturation at 94 °C for 30 s, annealing at 57 °C for 60 s and extension at 72 °C for 60 s, followed by a final extension for 10 min at 72 °C. The amplified PCR products were analyzed using 1.5% agarose gel with ethidium bromide (10 mg ml-1) stain. The gel was viewed under UV light using Alpha Imager HP gel documentation system (Alpha Innotech, USA).
Table 1
Evaluation of different genotypes of tomato against M. incognita (average of two-year data; 2016 and 2017)
RGI: Root Galling Index; Rf: Reproduction factor; Pf: Final nematode population; Pi: Initial nematode population; Initial population: 250 nematodes/250 g of soil. In reaction, R stands for Resistant; MR: Moderately resistant; S: Susceptible; and HS: Highly susceptible and the host susceptibility designation is by Begum et al. (2014). Values having * are significantly different from control (Susceptible check: Punjab Ratta) and “ns” indicates non-significance according to Dunnett test.
RESULTS AND DISCUSSION
Forty-seven genotypes of tomato were evaluated for their resistance against M. incognita both phenotypically and genotypically. The screening of genotypes in pot house revealed variable response to infestation of M. incognita. On the basis of phenotypic screening, the genotypes were grouped into four groups: resistant, moderately resistant, susceptible and highly susceptible (Table 1). Of these genotypes, only five were observed to be resistant. These were L-0272, NR-14, L-097, L-0275 and PNR-7. Root galling index was observed to be negligible in these genotypes (Table 1). Soil nematode population was also observed to decrease in pots of these genotypes after 60 days (Rf < 1) (Table 1). Among others, 13 genotypes (LO-125, VTG-68, BL-1200, PAU-ACC-1, CLN-104-48-1-0, LST-36-1, EC-531802, TBR-2, 102-8-5-1, Rakshita, 56-10-8-1, UHF-521 and L-3846) were found to be moderately resistant to M. incognita. Very few galls were observed on roots, with RGI ranging from 1.0-2.0. Nine genotypes viz. PAU-74, LST-17, CLN-6-7-0, Punjab Chhuhara, Angoorlata, KS-227, EC-535580, NR-5 and CLN-146A were found to be susceptible to M. incognita. The reproduction factor was higher in these genotypes as compared to resistant genotypes. More galls were observed on the roots of these genotypes (RGI: 2.1-3.0). Maximum M. incognita infestation was observed in highly susceptible genotypes viz. MLCR-3, Pant-T-11, Vellayani-Vijay, VFN-8, CLN-2714J, EC 15988, Azad-T-2, UTH-1-2, Malintka, TBR-1, Punjab Tropic, Haemshigga, Punjab Kesri, Punjab Upma, Swarna Lalima, CLN-37-8-1, EC-531804, Heelani, Russia and Punjab Ratta (susceptible check) with more than 70% of the roots infested and galled. Multiplication of root-knot nematode was maximum in these genotypes (2.06-2.68).
Among all the genotypes screened, maximum M. incognita population in soil was observed in cultivar EC-531804 (670 nematodes/250 cc soil) while minimum was found in genotype L-0275 (133 nematodes/250 cc soil). The soil nematode population in resistant genotypes was significantly reduced as compared to susceptible check (Punjab Ratta). Karssen and Moens (2006) reported that highly susceptible genotypes allowed the juveniles of nematodes to enter the roots, reached maturity and produced many eggs while resistant plants suppressed their development and thus do not allow reproduction. Significant increase in number of galls was observed in the susceptible genotypes as compared to resistant genotypes thereby affecting plant performance (Khan 1994). In the present study, it was also observed that the increase in nematode population in soil was more than two times when highly susceptible genotypes were grown as compared to the resistant genotypes.
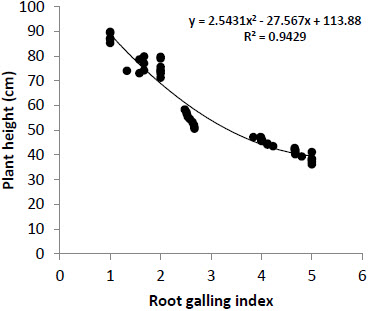
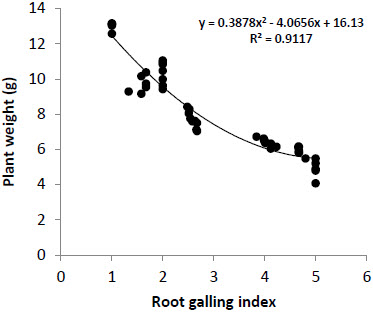
Growth parameters viz-a-viz. plant length (cm), shoot weight (g) and root weight (g) were also variably affected in different genotypes and were observed to be related to nematode infestation. There was a polynomial decrease in plant height and weight with increase in M. incognita infestation (Figs. 1, 2). Plant length was observed to be higher in resistant genotypes as compared to susceptible genotypes. Maximum plant height was observed in genotype NR-14 (89.83 cm) followed by PNR-7 (89.40 cm) and L-0275 (87.17 cm) whereas minimum plant height was recorded in genotype EC-531804 (36.20 cm). Shoot weight was also observed to be maximum in genotype NR-14 (25.18 g) followed by PNR-7 (24.92 g). Highly susceptible genotypes showed higher root weight which might be attributed to the increased weight of galls in roots.
Figure 1
Effect of Meloidogyneincognita infestation on plant height in 47 tomato genotypes exhibiting different level of susceptibility
Figure 2
Effect of Meloidogyneincognita infestation on plant weight in 47 tomato genotypes exhibiting different level of susceptibility
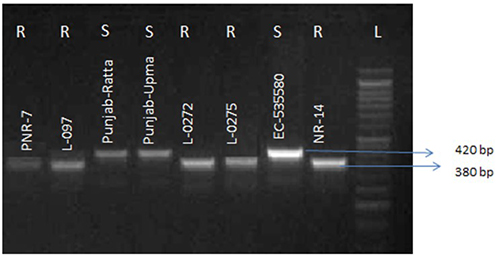
Figure 3
Agarose gel (1.5%) showing amplification of ~380 bp in resistant genotypes and ~420 bp in susceptible genotypes with Mi23 primer pair. (R = Resistant; S = Susceptible; L = DNA ladder 50 bp)
Molecular screening of the five resistant lines for presence of Mi gene using co-dominant SCAR Mi23 primer pair was done along with susceptible genotypes for revalidation of resistant genotypes. The DNA banding patterns of PCR ampli-fication products correlated well with the resistant and susceptible phenotypes obtained during phenotypic screening. In resistant genotypes (L-0272, NR-14, L-097, L-0275 and PNR-7), a ~380 bp amplicon was detected indicating the presence of Mi 1.2 gene conferring resistance against M. incognita. Whereas a ~420 bp PCR product was observed in the suscep-tible genotypes (Punjab Ratta, Punjab Upma and EC-535580) showing absence of the gene (Fig. 3). Danso et al. (2011) also observed that resistant phenotypes viz., VFNT, FLA 505-BL 1172, 2641A, “AdwoaDeede” and Terminator FI found during conventional screening when screened using Mi23 primer amplified a product of ~380 bp corresponding to Mi-1.2 gene. Mi23 is a co-dominant marker located within the Mi-1 locus and tightly linked to the Mi-1.2 gene (Seah et al. 2007). Until now, ten genes (Mi-1 to Mi-9 and Mi-HT) have been documented for resistance against Meloidogyne spp. (El-Sappah et al. 2019). Out of these, only Mi-1 has been commercially used for development of disease resistance in cultivated tomato (Ammiraju et al. 2003; Peng and Tang 2001). Use of molecular markers for marker-assisted selection for resistant genes greatly enhances the selection of resistant plants in breeding (Foolad and Panthee 2012).
Conclusively, from the genotypes screened, five sources of resistance viz. L-0272, NR-14, L-097, L-0275 and PNR-7 were identified against M. incognita with phenotypic screening which were revalidated by the use of molecular markers. Among the different management strategies being used for managing root-knot nematodes resistant breeding is the best alternative to manage these types of disease without affecting environment and living organisms. The resistant genotypes identified can be explored further for strengthening nematode resistance breeding programs of tomato against root-knot nematodes.
Appendices
ACKNOWLEDGEMENTS
Authors acknowledge the World Vegetable Center, AVRDC, Taiwan, for sharing of tomato genotypes (L-0272, L-0275, L-097 and L-3846) for the present study.
REFERENCES
- Ammiraju, J.S.S., J.C. Veremis, X. Huang, P.A. Roberts, and I. Kaloshian. 2003. The heat-stable root-knot nematode resistance gene Mi-9 from Lycopersicon peruvianum is localized on the short arm of chromosome 6. Theor. Appl. Genet. 106: 478-484.
- Anonymous. 2019. Tomato. Pages 33-41 in S.K. Thind, and J.S. Mahal (eds.), Package of practices for cultivation of vegetables. Punjab Agricultural University, Ludhiana, India.
- Begum, K., N. Hasan, S. Khandker, F.M. Aminuzzaman, M. Asaduzzaman, and N. Akhtar. 2014. Evaluation of brinjal cultivars (Solanum Melongena) against root-knot nematode Meloidogyne spp. Appl. Sci. Rep. 7: 129-134.
- Bozbuqa, R., H.Y. Dasgan, Y. Akhoundnejad, M. Imren, O.C. Gunay, and H. Toktay. 2020. Effect of Mi gene and nematode resistance on tomato genotypes using molecular and screening assay. Cytol. Genet. 54: 154-164.
- Cobb, N.A. 1918. Estimating the nematode population of a soil. Agricultural Technology Circular, Bureau of Plant Industry, Department of Agriculture, USA. 48 pp.
- Danso, Y., R. Akromah, and K. Osei. 2011. Molecular marker screening of tomato, (Solanum lycopersicum L.) germplasm for root-knot nematodes (Meloidogyne species) resistance. Afr. J. Biotechnol. 10: 1511-1515.
- den Ouden, H. 1958. A new method for culturing plants enabling observation of nematodes on growing roots. Eur. J. Plant Pathol. 64: 269-272.
- El-Sappah, A.H., M.M. Islam, H.H. El-awady, S. Yan, S. Qi, J. Liu, G. Cheng, and Y. Liang. 2019. Tomato natural resistance genes in controlling the root-knot nematode. Genes. 10. .
- Foolad, M.R., and D.R. Panthee. 2012. Marker-assisted selection in tomato breeding. Crit. Rev. Plant Sci. 31: 93-123. .
- Goggin, F.L., V.M. Williamson, and D.E. Ullman. 2001. Variability in the response of Macrosiphum euphorbiae and Myzus persicae (Hemiptera: Aphididae) to the tomato resistance gene Mi. Environ. Entomol. 30: 101-106.
- Indiastat. 2019. Socio-economic statistical information about India. Available online [http://www.indiastat.com] (Accessed in December 2019).
- Karssen, G., and M. Moens. 2006. Root-knot nematodes. Pages 59-90 in R.N. Perry, and M. Moens (eds.), Plant nematology. CABI publishing.
- Khan, M.R. 1994. Nematology in developing countries; India-IMP, Region VIII. Pages 379-398 in C.C. Carter, and J.N. Sasser (eds.), An advanced treatise on Meloidogyne vol. 1: Biology and control. Co-publication of Department of Plant Pathology North Carolina State University and the USAID, Raleigh, NC, USA.
- Lodhi, M.A., G. Ye, N.F. Weeden, and B.I. Reisch. 1994. A simple and efficient method for DNA extraction from grapevine cultivars and Vitis species. Plant Mol. Biol. Rep. 12: 6-13.
- Milligan, S.B., J. Bodeau, J. Yaghoobi, I. Kaloshian, P. Zabel, and V.M. Williamson. 1998. The root knot nematode resistance gene Mi from tomato is a member of the leucine zipper, nucleotide binding, leucine-rich repeat family of plant genes. Plant Cell. 10: 1307-1319.
- Peng, D., and W. Tang. 2001. Advance of resistance gene Mi to root knot nematodes in tomato. J. Shenyang Agric. Univ. 32: 220-223.
- Seah, S., A.C. Telleen, and V.M. Williamson. 2007. Introgressed and endogenous Mi-1 gene clusters in tomato differ by complex rearrangements in flanking sequences and show sequence exchange and diversifying selection among homologues. Theor. Appl. Genet. 114: 1289-1302.
- Seid, A., C. Fininsa, T. Mekete, W. Decraemer, and W.M.L. Wesemael. 2015. Tomato (Solanumlycopersicum) and root-knot nematodes (Meloidogyne spp.) – a century-old battle. Nematology. 17: 995-1009.
- Shurtleff, M.C., and C.W. Averre. 2000. Diagnosing plant disease caused by plant parasitic nematodes. The American Phytopathological Society, St-Paul, MN, USA. 187 pp.
- Taylor, A.L., and J.N. Sasser. 1978. Biology, identification and control of root-knot nematodes (Meloidogyne spp.). Department of Plant Pathology, North Carolina State University, United States Agency for International Development, Raleigh, NC, USA.
- Zakaria H.M., A.S. Kassab, M.M. Shamseldean, M.M. Oraby, and M.M.F. El-Mourshedy. 2013. Controlling the root-knot nematode, Meloidogyne incognita in cucumber plants using some soil bioagents and some amendments under simulated field conditions. Ann. Agric. Sci. 58: 77-82. .
List of figures
Figure 1
Effect of Meloidogyneincognita infestation on plant height in 47 tomato genotypes exhibiting different level of susceptibility
Figure 2
Effect of Meloidogyneincognita infestation on plant weight in 47 tomato genotypes exhibiting different level of susceptibility
Figure 3
Agarose gel (1.5%) showing amplification of ~380 bp in resistant genotypes and ~420 bp in susceptible genotypes with Mi23 primer pair. (R = Resistant; S = Susceptible; L = DNA ladder 50 bp)
List of tables
Table 1
Evaluation of different genotypes of tomato against M. incognita (average of two-year data; 2016 and 2017)
RGI: Root Galling Index; Rf: Reproduction factor; Pf: Final nematode population; Pi: Initial nematode population; Initial population: 250 nematodes/250 g of soil. In reaction, R stands for Resistant; MR: Moderately resistant; S: Susceptible; and HS: Highly susceptible and the host susceptibility designation is by Begum et al. (2014). Values having * are significantly different from control (Susceptible check: Punjab Ratta) and “ns” indicates non-significance according to Dunnett test.