Abstracts
Abstract
Wheat stripe rust (Puccinia striiforis f. sp. tritici) races CYR31 and CYR32, prevalent in China, are virulent to many wheat stripe rust resistance genes (Yr genes). To expand the availability of effective resistance to CYR31 and CYR32, stripe rust resistance was transferred from intermediate wheatgrass (Thinopyrum intermedium) to common wheat (Triticum aestivum). The susceptible wheat cultivar CM107 was crossed with amphiploid TAI7047, derived from the wide cross Taiyuan768/Thinopyrum intermedium//76(64). Two wheat lines originating from the cross, YU24 and YU25, were resistant to CYR31 and CYR32. Pedigree analysis showed that the resistance to stripe rust in YU24 and YU25 originated from intermediate wheatgrass. Genetic analyses indicated that the resistance to stripe rust is controlled by a single dominant gene. Allelic tests determined that the resistance gene(s) in YU24 and YU25 are identical. The new gene has temporarily been designated as YrYU25. SSR and RAPD analyses showed that YrYU25 was introduced by cryptic translocation into common wheat.
Keywords:
- Genetic resistance,
- intermediate wheatgrass,
- Puccinia striiformis f. sp. tritici,
- Thinopyrum intermedium,
- wheat
Résumé
Les races CYR31 et CYR32 de la rouille jaune du blé (Puccinia striiforis f. sp. tritici), très répandues en Chine, sont virulentes pour plusieurs gènes de résistance à cette maladie (gènes Yr). Afin d'accroître la disponibilité d'une résistance efficace aux races CYR31 et CYR32, la résistance à la rouille jaune du blé a été transférée de l'agropyre intermédiaire (Thinopyrum intermedium) au blé tendre (Triticum aestivum). CM107, un cultivar de blé sensible, a été croisé avec l'amphiploïde AI7047 dérivé du croisement éloigné Taiyuan768/Thinopyrum intermedium//76(64). Deux lignées de blé provenant de ce croisement, soit YU24 et YU25, étaient résistantes aux races CYR31 et CYR32. Une analyse généalogique a démontré que la résistance à la rouille jaune du blé chez les lignées YU24 et YU25 provenait de l'agropyre intermédiaire. Des analyses génétiques ont indiqué que cette résistance était contrôlée par un seul gène dominant. Des tests d'allélisme ont révélé que le(s) gène(s) de résistance dans les lignées YU24 et YU25 étaient identiques. Le nouveau gène a temporairement été nommé YrYU25. Des analyses SSR et RAPD ont démontré que le gène YrYU25 avait été introduit dans le blé tendre par translocation cryptique.
Mots clés:
- agropyre intermédiaire,
- blé,
- Puccinia striiformis f. sp. tritici,
- résistance génétique,
- Thinopyrum intermedium
Article body
Introduction
Stripe rust, caused by Puccinia striiformis Westend. f. sp. tritici Eriks., is still one of the most devastating diseases threatening wheat yield throughout the world, especially in cool and moist environments. The disease is controlled through resistance breeding and fungicides. Stripe rust attacks wheat at the early stages of plant development, and infection can result in stunted and weakened plants, leading to yield losses as high as 50% due to shriveled grain and damaged tillers (Roelfs et al. 1992). Although chemical control can reduce losses caused by stripe rust, and is the preferred means of control in some regions, genetic resistance remains a major objective for wheat breeding programs. The deployment of diverse resistance cultivars is the most effective, economical and environmentally-friendly approach for controlling this disease (Line and Chen 1995; Luo et al. 2009a). For more than half a century, wheat stripe rust has caused periodical epidemics and severe damage in China, especially in the southwest where the widely grown varieties have become susceptible. With the prevalence of the pathotypes CYR31 and CYR32, stripe rust has become the greatest threat to wheat yield because only a few of the known resistance genes are effective against these pathotypes (Luo et al. 2005, 2006, 2008a; Wan et al. 2004; Yang and Ren 2001). Long-lasting genetic resistance can be achieved through gene pyramiding (Johnson 1988), cultivar diversification, and cultivar mixing (Finckh 2008). Such strategies depend greatly on the availability of effective resistance genes. Thus, the identification of new stripe rust resistance genes is urgently needed to control the disease.
Intermediate wheatgrass Thinopyrum intermedium (Host) Barkworth and D.R. Dewey (2n = 6x = 42; JJJsJsSS) (syn. Elytrigia intermedia (Host) Nevski) has been hybridized extensively with wheat and has proven to be a useful source of disease resistance in hexaploid wheat (Triticum aestivum L.) (2n = 42; AABBDD) thanks to its close relationship with wheat. There is a lot of evidence suggesting that T. intermedium would constitute a potential tertiary gene pool for wheat resistance improvement to diseases such as wheat streak mosaic virus (Friebe et al. 1996), barley yellow dwarf virus (Ayala et al. 2001), Fusarium head blight (Fedak and Han 2005), leaf rust (Autrique et al. 1995), stem rust (Fedak 1999) and powdery mildew (Liu and Wang 2005; Liu et al. 2005). Recently, two powdery mildew resistance genes, Pm40 and Pm43, were transferred from Thinopyrum intermedium to common wheat by cryptic translocation, and they were located on chromosomal arm 7BS and 2DL, respectively (He et al. 2009; Luo et al. 2009b).
The main purpose of this study was to identify new genes for resistance to stripe rust by transferring such resistance from T. intermedium to common wheat. The introgressed resistance was then characterized through genetic analysis and molecular marker studies.
Materials and Methods
Virulence spectra of P. striiformis pathotypes CYR31 and CYR32
Various wheat differential lines (Table 1) carrying known Yr genes (kindly provided by Chen Xianming, Washington State University) as well as the main resistance genes utilized in the region were used to establish the virulence spectrum of the stripe rust pathotypes CYR31 and CYR32 and to differentiate the resistance response.
Table 1
Virulence testing of wheat stripe rust physiological strains CYR31 and CYR32 on stripe rust differential wheat lines and on main resistance cultivars in China
Genotype |
Genes |
Infection type (IT)a |
Season |
|
|---|---|---|---|---|
CYR31 |
CYR32 |
|||
Chinese 166 |
Yr1 |
4 |
4 |
Winter |
Leda |
Yr2 |
3 |
3 |
Winter |
Heines II |
Yr2, YrH II, Yr25 |
3 |
3 |
Winter |
Bon Fermier |
Yr3a |
4 |
4 |
Winter |
Capelle Desprez |
Yr3a, Yr4a, Yr16 |
3 |
3 |
Winter |
Avalon |
Yr3b, Yr4b, Yr14 |
4 |
4 |
Winter |
Minstre |
Yr3c, YrMin |
2 |
2 |
Winter |
Vilmorin 23 |
Yr4a, YrV23 |
3 |
3 |
Winter |
Hybrid 46 |
Yr4b, YrH46 |
3 |
3 |
Winter |
Opal |
Yr4b |
3 |
3 |
Spring |
AVS/6* Yr5 |
Yr5 |
0 |
0+ |
Spring |
T. spelta var. album |
Yr5 |
0 |
0 |
Winter |
Fielder |
Yr6, Yr20 |
3 |
4 |
Spring |
Heines Kolben |
Yr6, YrHK |
4 |
4 |
Winter |
Lee |
Yr7, Yr22, Yr23 |
3 |
4 |
Spring |
Thatcher |
Yr7 |
4 |
4 |
Spring |
Maris Widgeon |
Yr8 |
3 |
4 |
Winter |
Compair |
Yr8, Yr19 |
1 |
1 |
Spring |
Aurora |
Yr9 |
3 |
3 |
Winter |
Benno |
Yr9 |
3 |
3 |
Winter |
Moro |
Yr10, YrMor |
0+ |
0+ |
Winter |
PI178383 |
Yr10 |
0+ |
0+ |
Winter |
Joss Cambier |
Yr11 |
3 |
3 |
Winter |
Mega |
Yr3a, Yr4a, Yr12 |
3 |
3 |
Winter |
Armada |
Yr3a, Yr4a, Yr12 |
3 |
3 |
Winter |
Mardler |
Yr1, Yr2, Yr3a, Yr4a, Yr13 |
1 |
1 |
Winter |
Kador |
Yr14 |
3 |
3 |
Winter |
AVS/6* Yr15 |
Yr15 |
0+ |
0+ |
Spring |
Hybrid de Bersee |
Yr16 |
3 |
3 |
Winter |
AVS/6* Yr17 |
Yr17 |
3 |
3 |
Spring |
Jupetico R |
Yr18 |
3 |
3 |
Spring |
Lemhi |
Yr21 |
4 |
4 |
Spring |
Yr24 |
Yr24 |
4 |
4 |
Spring |
TP981 |
Yr25 |
4 |
4 |
Winter |
Yr26 |
Yr26 |
3 |
4 |
Spring |
R212 |
YrR212 |
0 |
0 |
Winter |
AIM6 |
YrCN19 |
0 |
0 |
Winter |
R185 |
YrR212 |
0 |
0 |
Winter |
Ciano79 |
Yr27 |
3 |
3 |
Winter |
Pastor |
Yr31 |
3 |
3 |
Winter |
R88 |
YrCN19 |
0 |
0 |
Winter |
R57 |
YrCN17 |
1 |
1 |
Winter |
R59 |
YrCN17 |
1 |
1 |
Winter |
R25 |
YrCN17 |
1 |
1 |
Winter |
AIM5 |
YrCN19 |
0 |
0 |
Winter |
Spolding Prolifie (M) |
Yrsp |
3 |
3 |
Winter |
0 = no visible symptoms; 0+ = visible necrotic flecks without uredia; 1 = small sporulating uredia surrounded by necrotic tissue; 2 = small-size uredia with chlorosis and necrosis; 3 = moderately-sized sporulating uredia surrounded only by chlorotic tissue; and 4 = abundantly sporulating uredia without chlorosis.
Introgression and genetic analysis of stripe rust resistance
Chuanmai107 (CM107) was pollinated with octoploid Trititrigia TAI7047, derived from the wide cross Taiyuan768/T. intermedium//76(64). Two wheat lines, Yuan24 (YU24) and Yuan25 (YU25) (Triticum aestivum, 2n = 6x = 42, AABBDD), were selected from the F5 population of the cross (CM107/TAI7047) (Luo et al. 2009b). The two lines were characterized by a hypersensitive response to both races of P. striiformis f. sp. Tritici, CYR31 and CYR32, following natural infection. To study the genetic composition and nature of the resistance, three F2 populations were derived from the F1 population originating from the crosses MY11/YU24, MY11/YU25 and YU24/YU25. The F1 hybrids were also backcrossed to both parents. The backcross to the susceptible parent (BC1SF1) and the backcross to the resistant parent (BC1RF1) were allowed to self-pollinate, and both sets of F1 and F2 populations were genetically analyzed.
Stripe rust resistance screening
Puccinia striiformis f. sp. tritici pathotypes CYR31 and CYR32, which are the predominant strains in southwest China, were used to screen and assess the resistance response of homogeneous genotypes. To test the resistance of segregating populations, plants were inoculated with CYR32 because it has the same virulent factors as CYR31 (Wan et al. 2004).
The various wheat genotypes, including differential lines, main resistance lines and parental lines, were grown in a temperature- and moisture-controlled glass enclosure (25 m x 10 m; 1.6 m in height) at the Experimental Station of Sichuan Agriculture University. At the three-leaf stage, 20 seedlings per genotype were inoculated with single isolates of both CYR31 and CYR32 according to Luo et al. (2005). The inoculated seedlings were misted through an inlet until sufficient dew was formed and incubated at 14°C in the dark for 12 h and in the light for 24 h. They were then grown under natural daylight at 16-20°C. When the stripe rust pustules were fully developed, the infection types (ITs) were recorded on a 0 to 4 scale described by Wellings et al. (1988): IT0 = no visible symptoms; IT0+ = visible necrotic flecks without uredia; IT1 = small sporulating uredia surrounded by necrotic tissue; IT2 = small-size uredia with chlorosis and necrosis; IT3 = moderately-sized sporulating uredia surrounded only by chlorotic tissue; and IT4 = abundantly sporulating uredia without chlorosis.
Genomic DNA extraction and analysis
DNA was extracted using 1 g of fresh wheat leaves from 5-wk-old seedlings (Tai and Tanksley 1990). DNA of intermediate wheatgrass and of wheat genotypes Chinese Spring (CS), MY11, CM107, YU24, YU25 and octoploid Trititrigia TAI7047 was used to screen for the presence of foreign DNA segments using wheat-specific microsatellites markers (SSR) and random amplified polymorphic DNA (RAPD). For SSR analysis, PCR reactions were carried out in an MJ RESEARCH (PTC-200) thermocycler using publicly available Xgwm primer pairs. For each PCR reaction, the 20 µL volume mixture contained 200 nM of each primer, 0.2 mM deoxynucleotides, 50 mM KCl, 10 mM Tris-HCl, 1.5 mM MgCl2, 1 unit Taq polymerase (Pharmacia) and 50 ng template DNA. After 3 min denaturation at 94°C, 43 cycles were performed with 1 min denaturation at 94°C, 1 min annealing at temperatures varying from 50-60°C (depending on the primer sequence), and a 2 min extension at 72°C. A final extension step of 10 min at 72°C was performed (Roder et al. 1998).
RAPD analysis was performed in 20 µL reaction volumes as described by Williams et al. (1990). In total, 520 primers were screened from Operon primer kits A-Z. The amplified fragments were run on 3% agarose (FMC brand) in 0.5X TBE at a voltage of 120 V (4V cm‑1) and were then visualized using ethidium bromide staining methods.
Results
Virulence of P. striiformis pathotypes CYR31 and CYR32 on main known resistance genes
Inoculation tests showed that CYR31 and CYR32 are virulent on most of the described wheat stripe rust resistance genes (Table 1). CYR31 and CYR32 are virulent to Yr1, Yr2, Yr3a, Yr3b, Yr3c, Yr4a, Yr4b, Yr6, Yr7, Yr8, Yr9, Yr11, Yr12, Yr14, Yr16, Yr17, Yr18, Yr20, Yr21, Yr22, Yr23, Yr24, Yr25, Yr26, Yr27, Yr31, YrHVII, YrMin, YrV23, Yrsp, YrH46 and YrHK. Only Yr5, Yr10, Yr15, YrCN19 and YrR212 were effective against CYR31 and CYR32, while Yr13, Yr19 and YrCN17 were partially effective (Table 1).
The resistance response of different genotypes to CYR31 and CYR32
The parental genotypes CS, CM107, MY11, Taiyuan768 and 76(64) were susceptible, with high infection types ranging between 3 and 4. Thinopyrum intermedium wheatgrass, YU24, YU25 and TAI7047 were resistant, with low infection type IT=0+ (Table 2). Their leaves produced obvious large necrotic lesions between the veins of adult plants after inoculation (Fig. 1).
Table 2
Reaction of various wheat and intra-specific hybrids to Puccinia striiformis f. sp. tritici physiological strains CYR31 and CYR32
Genotype |
Pedigree |
Infection type (IT)a |
|
|---|---|---|---|
CYR31 |
CYR32 |
||
CS |
|
3 |
3 |
MY11 |
|
4 |
4 |
YU24 |
CM107/TAI7047 |
0+ |
0+ |
YU25 |
CM107/TAI7047 |
0+ |
0+ |
CM107 |
|
4 |
4 |
TAI7047 |
Taiyuan768/Th. intermedium//76(64) |
0+ |
0+ |
Intermediate wheatgrass |
|
0 |
0 |
Taiyuan768 |
|
3 |
4 |
76(64) |
|
4 |
4 |
0 = no visible symptoms; 0+ = visible necrotic flecks without uredia; 1 = small sporulating uredia surrounded by necrotic tissue; 2 = small-size uredia with chlorosis and necrosis; 3 = moderately-sized sporulating uredia surrounded only by chlorotic tissue; and 4 = abundantly sporulating uredia without chlorosis.
Figure 1
The different infection types of wheat stripe rust resistance to physiological strain CYR32 in various wheat genotypes.
Genetic analyses of Thinopyrum intermedium-derived stripe rust resistance
The data obtained following evaluation of the seedlings of parental lines and cross progenies with isolates CYR31 and CYR32 are summarized in Table 2. Towards the end of growth, there were still no visible symptoms on the leaves of intermediate wheatgrass. The F1 MY11/YU24 and MY11/YU25 were also highly resistant (Table 3), indicating the presence of one or more dominant resistance genes in YU24 and YU25. The two F2 populations (crosses MY11/YU24 and MY11/YU25) each showed 3 resistant:1 susceptible segregation of a single dominant resistance gene (Table 3). Single gene segregation was also confirmed in the two BC1SF1 populations, which fitted the expected 1:1 ratio. Segregation in the backcross F2 populations also fitted the expected segregation of a single dominant resistance gene. All F1 and F2 populations produced from the cross YU24/YU25 were resistant (Tables 2 and 3), suggesting that the same gene was present in both parents.
Table 3
Resistance segregation of YU24 and Yu25 in various genetic backgrounds
Pedigree |
Generation |
CYR32 |
Expected ratio |
χ2 |
P |
|
|---|---|---|---|---|---|---|
R |
S |
|||||
MY11/YU25 (F1) |
F1 |
21 |
0 |
|
|
|
MY11/YU24 (F1) |
F1 |
19 |
0 |
|
|
|
YU24/YU25 (F1) |
F1 |
23 |
0 |
|
|
|
MY11/Yu25 |
F2 |
136 |
47 |
3:1 |
0.05 |
0.83 |
MY11/YU24 |
F2 |
133 |
39 |
3:1 |
0.50 |
0.48 |
MY11/YU25//YU25 |
BC1RF1a |
68 |
|
|
|
|
|
BC1RF2 |
128 |
22 |
7:1 |
0.64 |
0.42 |
MY11/YU24//YU24 |
BC1RF1 |
73 |
|
|
|
|
|
BC1RF2 |
132 |
23 |
7:1 |
0.78 |
0.38 |
MY11/YU25//MY11 |
BC1SF1a |
41 |
46 |
1:1 |
0.29 |
0.59 |
|
BC1SF2 |
64 |
112 |
3:5 |
0.10 |
0.76 |
MY11/YU24//MY11 |
BC1SF1 |
39 |
44 |
1:1 |
0.30 |
0.58 |
|
BC1SF2 |
59 |
104 |
3:5 |
0.12 |
0.73 |
YU25/YU24 |
F2 |
253 |
0 |
|
|
|
R and S refer to backcrosses made to the resistant and susceptible parents, respectively.
Determination of foreign DNA segments in YU24 and YU25
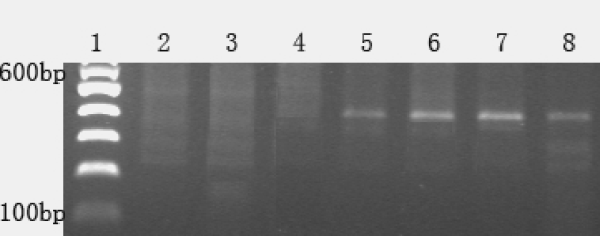
In an attempt to determine the chromosome location of the introgressed foreign chromatin, 294 pairs of wheat microsatellite primers that amplify repeat sequences distributed over all wheat chromosomes were used. The results showed that wheat genotypes YU24, YU25 and octoploid TAI7047, as well as common wheat controls MY11, CM107 and CS, amplified wheat-specific SSR products that did not occur in intermediate wheatgrass. In addition, 520 primers (Operon groups A to Z) were used in an attempt to identify amplicons specific to intermediate wheatgrass. Thirteen RAPD primers (B2, F9, I17, M8, N11, P6, Q18, R1, R10, R16, R19, U5 and Z20) produced amplified products in wheat genotypes YU24 and YU25 and octoploid Trititrigia TAI7047, and these products had the same length as those in intermediate wheatgrass. The PCR results amplified by R1 are shown in Figure 2.
Figure 2
PCR results of various genomic DNA amplified by RAPD primer R1.
Lane 1 = Marker; 2 = Chinese Spring; 3 = MY11; 4 = CM107; 5 = TAI7047; 6 = E. intermedium; 7 = YU24; 8 = YU25.
Discussion
Although many stripe rust resistance genes have been identified to date and incorporated into high-yield wheat cultivars, most of them are no longer effective against the stripe rust pathotypes prevalent in southwest China (Wan et al. 2004; Yang et al. 2003). Wheat lines YU24 and YU25 have a high level of resistance (IT = 0+) to stripe rust pathotypes CYR31 and CYR32, which was associated with large necrotic spots on the leaves (Table 2 and Fig. 1). Although the genotypes Moro (Yr10 and YrMor), PI178383 (Yr10) and AVS/6*Yr15 (Yr15) had similar infection types (IT = 0+), they produced less necrosis. Moreover, YU24 and YU25 were derived from a wide cross between wheat cultivar CM107 and octoploid Trititrigia TAI7047 (Taiyuan768/Th. intermediate wheatgrass//74(64)). In these pedigrees, all common wheat lines were susceptible to stripe rust pathotypes CYR31 and CYR32 (Table 2); hence, the resistance factors were derived from intermediate wheatgrass and they therefore represent a new source of wheat stripe rust resistance. This also confirms that intermediate wheatgrass is an accessible source of disease resistance genes.
Genetic analysis of the resistance in YU24 and YU25 showed that segregation in the segregating populations complied with the expected segregation as a single dominant factor (Table 2), thus suggesting the presence of a dominant resistance gene. The results of allelic tests for resistance (Tables 2 and 3) revealed that the resistance genes to stripe rust in both parents are identical. None of the resistance genes published for wheat stripe rust originated from intermediate wheatgrass (Luo et al. 2008b), and the stripe rust resistance gene in YU24 and YU25 is different from the wheat stripe rust resistance genes published so far; it is therefore a new gene, which has temporarily been designated as YrYU25.
Chromosomal translocation is a classic and useful method for transferring alien genes from wild relatives to common wheat (Ren and Zhang 1997). Most of these translocations, despite carrying useful alien genes, have a questionable value for wheat improvement because the large transferred chromosome segments do not adequately compensate for the wheat genes they replace or they carry additional genes conferring undesirable traits. However, in a few instances, traits of interest were transferred to recipient genotypes without inducing detectable cytological or genetic changes (Dong et al. 2004; He et al. 2009; Kuraparthy et al. 2007; Luo et al. 2009b; Multani et al. 1994; Ren and Zhang 1997). The results of the SSR analysis showed that YU24 and YU25 amplified wheat-specific products evenly distributed over all chromosomal arms. This indicates that there is no whole foreign chromosomal arm in YU24 and YU25. Previous cytological studies have shown that YU24 and YU25 are genetically stable (2n = 42, 21II), and agronomical trait observation over the past several years has also proven that they are homogeneous (Luo et al. 2009b; Ma et al. 2007). The resistance segregation of YU24 and YU25 behaved as a normal Mendelian unit. Moreover, we could not detect hybridization signals in situ using Th. intermedium genomic DNA as a probe (data not shown). This evidence persuaded us to conclude that wheat resistance genotypes YU24 and YU25 do not have an entire foreign chromosomal arm and, therefore, that the new stripe rust resistance source could be used to expand resistance genetic diversity in common wheat.
Appendices
Acknowledgements
We are grateful to the National Natural Science Foundation of China (No. 30971787) and the Fok Ying Tung Educations Foundation (No. 111030). We are also grateful to Dr. R.A. McIntosh, University of Sydney, for providing the seeds Spolding Prolifie, and to Dr. X.M. Chen, Washington State University, for providing the seeds with Yr genotypes.
References
- Autrique, E., R.P. Singh, S.D. Tanksley, and M.E. Sorrells. 1995. Molecular markers for four leaf rust resistance genes introgressed into wheat from wild relatives. Genome 38 : 75-83.
- Ayala, L., M. Henry, D. Gonzalez-de-Leon, M. van Ginkel, A. Mujeeb-Kazi, B. Keller, and M. Khairallah. 2001. A diagnostic molecular marker allowing the study of Th. intermedium-derived resistance to BYDV in bread wheat segregating populations. Theor. Appl. Genet. 102 : 942-949.
- Dong, Y.S., X.L. Bu, Y.S. Luan, M.Y. He, and B. Liu. 2004. Molecular characterization of a cryptic wheat – Thinopyrum intermedium translocation line: evidence for genomic instability in nascent allopolyploid and aneuploid lines. Genet. Mol. Biol. 27 : 237-241.
- Fedak, G. 1999. Molecular aids for integration of alien chromatin through wide crosses. Genome 42 : 584-591.
- Fedak, G. and F. Han. 2005. Characterization of derivatives from wheat-Thinopyrum wide crosses. Cytogenet. Genome Res. 109 : 360-367.
- Finckh, M.R. 2008. Integration of breeding and technology into diversification strategies for disease control in modern agriculture. Eur. J. Plant Pathol. 121 : 399-409.
- Friebe, B., J. Jiang, W.J. Raupp, R.A. McIntosh, and B.S. Gill. 1996. Characterization of wheat-alien translocations conferring resistance to diseases and pests: current status. Euphytica 91 : 59-87.
- He, R., Z. Chang, Z. Yuan, H. Zhan, X. Zhang, and J. Liu. 2009. Inheritance and mapping of powdery mildew resistance gene Pm43 introgressed from Thinopyrum intermedium into wheat. Theor. Appl. Genet. 118 : 1173-1180.
- Johnson, R. 1988. Durable resistance to yellow (stripe) rust in wheat and its implications in plant breeding. Pages 63-75 in N.W. Simmonds and S. Rajaram (eds.), Breeding Strategies for Resistance to the Rusts of Wheat. CIMMYT, Mexico.
- Kuraparthy, V., S. Sood, P. Chhuneja, H.S. Dhaliwal, S. Kaur, R.L. Bowder, and B.S. Gill. 2007. A cryptic wheat-Aegilops triuncialis translocation with leaf rust resistance gene Lr58. Crop Sci. 47 : 1995-2003.
- Line, R.F., and X.M. Chen. 1995. Success in breeding for and managing durable resistance to wheat rusts. Plant Dis. 79 : 1254-1255.
- Liu, S.B., and H.G. Wang. 2005. Characterization of wheat-Thinopyron intermedium substitution line with resistance to powdery mildew. Euphytica 143 : 229-233.
- Liu, S.B., H.G. Wang, X.Y. Zhang, X.F. Li, D.Y. Li, X.Y. Duan, and Y.L. Zhou. 2005. Molecular cytogenetic identification of a wheat-Thinopyron intermedium (Host) Barkworth & DR Dewey partial amphiploid resistant to powdery mildew. J. Integr. Plant Biol. 47 : 726-733.
- Luo, P.G., Z.L. Ren, H.Q. Zhang, and H.Y. Zhang. 2005. Identification, chromosomal location, and diagnostic markers for a new gene (YrCN19) for resistance to wheat stripe rust. Phytopathology 95 : 1266-1270.
- Luo, P.G., Z.L. Ren, H.Q. Zhang, and H.Y. Zhang. 2006. Diagnostic detection and genetic analysis of wheat stripe rust resistant gene YrCN19. J. Mol. Cell Biol. 39 : 217-222.
- Luo, P.G., H.Y. Zhang, K. Shu, H.Y. Zhang, and Z.L. Ren. 2008a. Diversity of stripe rust (Puccinia striiformis f. sp. tritici) resistance in wheat genotype with 1RS chromosomal translocations from different rye lines. Can. J. Plant Pathol. 30 : 254-259.
- Luo, P.G., X.Y. Hu, H.Y. Zhang, H.Q. Zhang, and Z.L. Ren. 2008b. Allelic analysis of stripe rust resistance genes on wheat chromosome 2BS. Genome 51 : 922-927.
- Luo, P.G., X.Y. Hu, H.Y. Zhang, and Z.L. Ren. 2009a. Genes for resistance to stripe rust on chromosome 2B and their application in wheat breeding. Prog. Nat. Sci. 19 : 9-15.
- Luo P.G., H.Y. Luo, Z.J. Chang, H.Y. Zhang, and Z.L. Ren. 2009b. Characterization and chromosomal location Pm40 in common wheat: a new gene for resistance to powdery mildew derived from Elytrigia intermedium. Theor. Appl. Genet. 118 : 1059-1064.
- Ma, Q., P.G. Luo, Z.L. Ren, H.R. Jiang, and Z.J. Yang. 2007. Genetic analysis and chromosomal location of two new genes for resistance to powdery mildew in wheat (Triticum aestivum L.). Acta Agric. Sin. 33 : 1-8.
- Multani, D.S., K.K. Jena, and D.S. Brar. 1994. Development of monosomic alien addition lines and introgression of genes from Oryza australiensis domain to cultivated rice Oryza sativa L. Theor. Appl. Genet. 88 : 102-109.
- Ren, Z.L., and H.Q. Zhang. 1997. Induction of small-segment-translocation between wheat and rye chromosomes. Sci. China (Series C) 40 : 323-331.
- Roder, M.S., V. Korzun, K. Wendehake, J. Plaschke, M.H. Tixier, P. Leroy, and M.W. Ganal. 1998. A microsatellite map of wheat. Genetics 149 : 1105-1114.
- Roelfs, A.P., R.P. Singh, and E.E. Saari. 1992. Rust Disease of Wheat: Concept and Methods of Disease Management. CIMMYT, Mexico, D.-F. 81 p.
- Tai, T.H., and S.D. Tanksley. 1990. A rapid and inexpensive method for isolation of total DNA from dehydrated plant tissue. Plant Mol. Biol. Rep. 8 : 297-303.
- Wan, A.M., Z.H. Zhao, X.M. Chen, Z.H. He, S.L. Jin, Q.Z. Jia, G. Yao, J.X. Yang, B.T. Wang, G.B. Li, Y.Q. Bi, and X.Y. Yuan. 2004. Wheat stripe rust epidemic and virulence of Puccinia striiformis f. sp. tritici in China in 2002. Plant Dis. 88 : 896-904.
- Wellings, C.R., R.A. McIntosh, and M. Hussain. 1988. A new source of resistance to Puccinia striiformis f.sp. tritici in spring wheats. Plant Breed. 110 : 88-96.
- Williams, J.G.K., A.R. Kubelik, J.A. Livak, J.A. Rafalski, and S.V. Tingey. 1990. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res. 18 : 6531-6535.
- Yang, Z.J., and Z.L. Ren. 2001. Chromosomal distribution and genetic expression of Lophopyrum elongatum (Host) A. Löve genes for adult plant resistance to stripe rust in wheat background. Genet. Resour. Crop Evol. 48 : 183-187.
- Yang, Z.M., C.J. Xie, and Q.X. Sun. 2003. Situation of the sources of stripe rust resistance of wheat in the post-CY32 era. Acta Agric. Sin. 29 : 161-168.
List of figures
Figure 1
The different infection types of wheat stripe rust resistance to physiological strain CYR32 in various wheat genotypes.
Figure 2
PCR results of various genomic DNA amplified by RAPD primer R1.
List of tables
Table 1
Virulence testing of wheat stripe rust physiological strains CYR31 and CYR32 on stripe rust differential wheat lines and on main resistance cultivars in China
Genotype |
Genes |
Infection type (IT)a |
Season |
|
|---|---|---|---|---|
CYR31 |
CYR32 |
|||
Chinese 166 |
Yr1 |
4 |
4 |
Winter |
Leda |
Yr2 |
3 |
3 |
Winter |
Heines II |
Yr2, YrH II, Yr25 |
3 |
3 |
Winter |
Bon Fermier |
Yr3a |
4 |
4 |
Winter |
Capelle Desprez |
Yr3a, Yr4a, Yr16 |
3 |
3 |
Winter |
Avalon |
Yr3b, Yr4b, Yr14 |
4 |
4 |
Winter |
Minstre |
Yr3c, YrMin |
2 |
2 |
Winter |
Vilmorin 23 |
Yr4a, YrV23 |
3 |
3 |
Winter |
Hybrid 46 |
Yr4b, YrH46 |
3 |
3 |
Winter |
Opal |
Yr4b |
3 |
3 |
Spring |
AVS/6* Yr5 |
Yr5 |
0 |
0+ |
Spring |
T. spelta var. album |
Yr5 |
0 |
0 |
Winter |
Fielder |
Yr6, Yr20 |
3 |
4 |
Spring |
Heines Kolben |
Yr6, YrHK |
4 |
4 |
Winter |
Lee |
Yr7, Yr22, Yr23 |
3 |
4 |
Spring |
Thatcher |
Yr7 |
4 |
4 |
Spring |
Maris Widgeon |
Yr8 |
3 |
4 |
Winter |
Compair |
Yr8, Yr19 |
1 |
1 |
Spring |
Aurora |
Yr9 |
3 |
3 |
Winter |
Benno |
Yr9 |
3 |
3 |
Winter |
Moro |
Yr10, YrMor |
0+ |
0+ |
Winter |
PI178383 |
Yr10 |
0+ |
0+ |
Winter |
Joss Cambier |
Yr11 |
3 |
3 |
Winter |
Mega |
Yr3a, Yr4a, Yr12 |
3 |
3 |
Winter |
Armada |
Yr3a, Yr4a, Yr12 |
3 |
3 |
Winter |
Mardler |
Yr1, Yr2, Yr3a, Yr4a, Yr13 |
1 |
1 |
Winter |
Kador |
Yr14 |
3 |
3 |
Winter |
AVS/6* Yr15 |
Yr15 |
0+ |
0+ |
Spring |
Hybrid de Bersee |
Yr16 |
3 |
3 |
Winter |
AVS/6* Yr17 |
Yr17 |
3 |
3 |
Spring |
Jupetico R |
Yr18 |
3 |
3 |
Spring |
Lemhi |
Yr21 |
4 |
4 |
Spring |
Yr24 |
Yr24 |
4 |
4 |
Spring |
TP981 |
Yr25 |
4 |
4 |
Winter |
Yr26 |
Yr26 |
3 |
4 |
Spring |
R212 |
YrR212 |
0 |
0 |
Winter |
AIM6 |
YrCN19 |
0 |
0 |
Winter |
R185 |
YrR212 |
0 |
0 |
Winter |
Ciano79 |
Yr27 |
3 |
3 |
Winter |
Pastor |
Yr31 |
3 |
3 |
Winter |
R88 |
YrCN19 |
0 |
0 |
Winter |
R57 |
YrCN17 |
1 |
1 |
Winter |
R59 |
YrCN17 |
1 |
1 |
Winter |
R25 |
YrCN17 |
1 |
1 |
Winter |
AIM5 |
YrCN19 |
0 |
0 |
Winter |
Spolding Prolifie (M) |
Yrsp |
3 |
3 |
Winter |
0 = no visible symptoms; 0+ = visible necrotic flecks without uredia; 1 = small sporulating uredia surrounded by necrotic tissue; 2 = small-size uredia with chlorosis and necrosis; 3 = moderately-sized sporulating uredia surrounded only by chlorotic tissue; and 4 = abundantly sporulating uredia without chlorosis.
Table 2
Reaction of various wheat and intra-specific hybrids to Puccinia striiformis f. sp. tritici physiological strains CYR31 and CYR32
Genotype |
Pedigree |
Infection type (IT)a |
|
|---|---|---|---|
CYR31 |
CYR32 |
||
CS |
|
3 |
3 |
MY11 |
|
4 |
4 |
YU24 |
CM107/TAI7047 |
0+ |
0+ |
YU25 |
CM107/TAI7047 |
0+ |
0+ |
CM107 |
|
4 |
4 |
TAI7047 |
Taiyuan768/Th. intermedium//76(64) |
0+ |
0+ |
Intermediate wheatgrass |
|
0 |
0 |
Taiyuan768 |
|
3 |
4 |
76(64) |
|
4 |
4 |
0 = no visible symptoms; 0+ = visible necrotic flecks without uredia; 1 = small sporulating uredia surrounded by necrotic tissue; 2 = small-size uredia with chlorosis and necrosis; 3 = moderately-sized sporulating uredia surrounded only by chlorotic tissue; and 4 = abundantly sporulating uredia without chlorosis.
Table 3
Resistance segregation of YU24 and Yu25 in various genetic backgrounds
Pedigree |
Generation |
CYR32 |
Expected ratio |
χ2 |
P |
|
|---|---|---|---|---|---|---|
R |
S |
|||||
MY11/YU25 (F1) |
F1 |
21 |
0 |
|
|
|
MY11/YU24 (F1) |
F1 |
19 |
0 |
|
|
|
YU24/YU25 (F1) |
F1 |
23 |
0 |
|
|
|
MY11/Yu25 |
F2 |
136 |
47 |
3:1 |
0.05 |
0.83 |
MY11/YU24 |
F2 |
133 |
39 |
3:1 |
0.50 |
0.48 |
MY11/YU25//YU25 |
BC1RF1a |
68 |
|
|
|
|
|
BC1RF2 |
128 |
22 |
7:1 |
0.64 |
0.42 |
MY11/YU24//YU24 |
BC1RF1 |
73 |
|
|
|
|
|
BC1RF2 |
132 |
23 |
7:1 |
0.78 |
0.38 |
MY11/YU25//MY11 |
BC1SF1a |
41 |
46 |
1:1 |
0.29 |
0.59 |
|
BC1SF2 |
64 |
112 |
3:5 |
0.10 |
0.76 |
MY11/YU24//MY11 |
BC1SF1 |
39 |
44 |
1:1 |
0.30 |
0.58 |
|
BC1SF2 |
59 |
104 |
3:5 |
0.12 |
0.73 |
YU25/YU24 |
F2 |
253 |
0 |
|
|
|
R and S refer to backcrosses made to the resistant and susceptible parents, respectively.